00:41

China's self-developed drug for Alzheimer's Disease (AD) has been greenlighted for sales on Saturday, filling a 17-year blank in this field.
The National Medical Products Administration (NMPA) approved the marketing application of a Shanghai-based company for their new treatment, Oligomannate, known as GV-971, which is expected to improve cognitive function in mild-to-moderate AD patients, according to a statement on NMPA's website.
But further research on Oligomannate's pharmacological mechanism and long-term safety and effectiveness is required, the NMPA statement wrote.
Screenshot from China's NMPA
Screenshot from China's NMPA
"Any company that can develop a drug to slow the progression of AD would be a huge blow to its global competitors," Wu Shenghu, Lilly's medical director of neuroscience told China Business Network.
Shanghai Green Valley Pharmaceutical Company and other two academic institutions in China jointly developed the drug, GV-971. It is a low molecular acid oligosaccharide compound extracted from marine brown algae. The company claimed that trial results demonstrated improved patients' cognitive function as early as week 4 and the benefit was sustained at each follow-up assessment visit.
"The current drug treatment of AD is still symptomatic, and there are few drugs available to delay or prevent the progression of the disease. With the new mechanism clinical effects of GV-971, it is believed that this drug can provide a new solution for the treatment," Professor Xiao Shifu, co-lead researcher of the drug explained.
AD patients are usually diagnosed with cognitive and behavioral disorders, as well as some mental abnormality. It is the third major disease causing disability and death among elderly after cardiovascular diseases and malignant tumors.
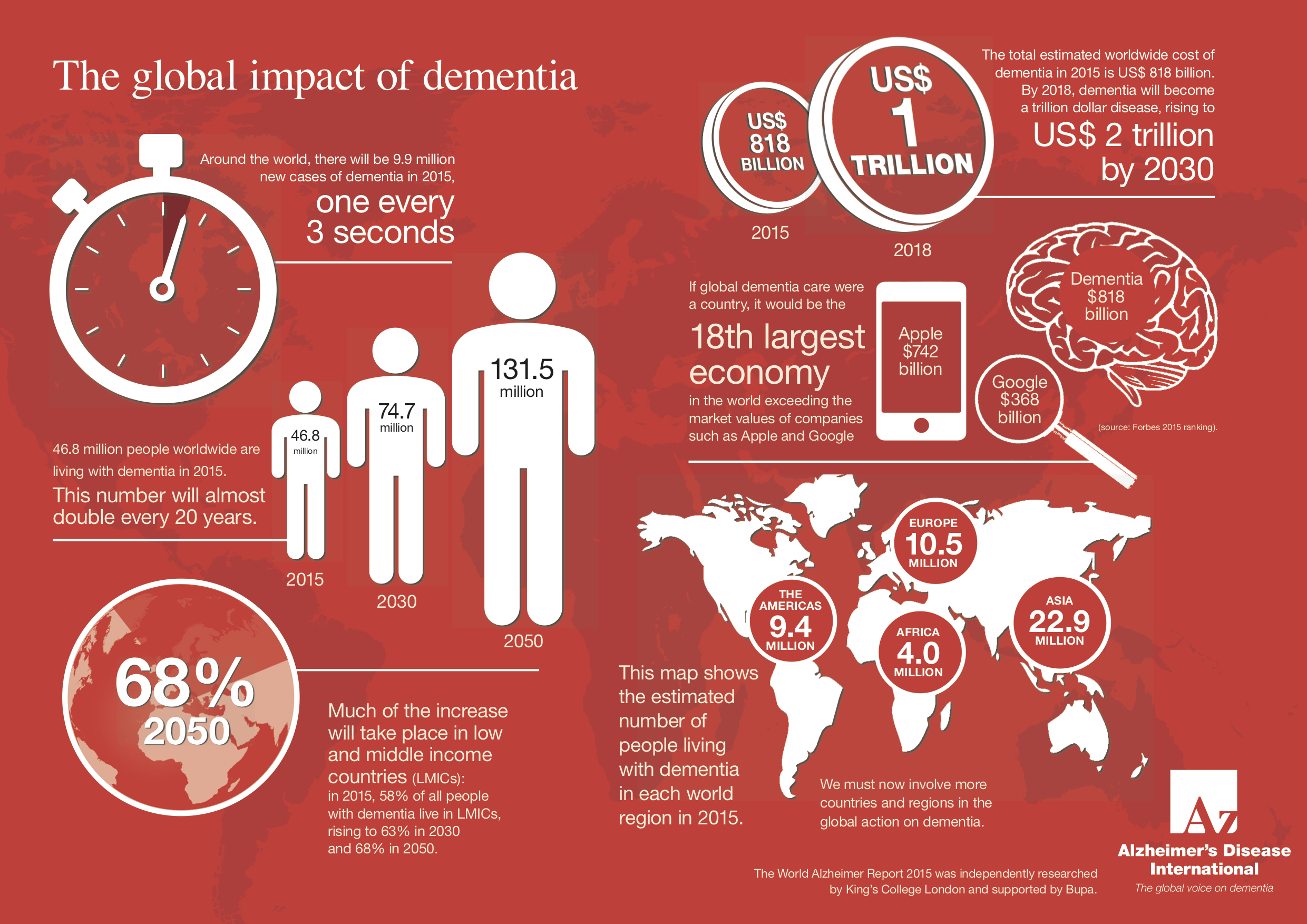
Besides, AD is also the most common cause of dementia among older adults. According to World Health Organization, around 50 million people have dementia, and there are nearly 10 million new cases every year. It is estimated that 75 million people will suffer from dementia in 2030 and the number will reach 131.5 million in 2050. The majority of patients are from developing countries.
Infographic via Alzheimer's Disease International
Infographic via Alzheimer's Disease International
Since Dr. Alois Alzheimer discovered the disease in 1906, only several prescription drugs have been approved by the U.S. Food and Drug Administration (FDA), but the results are not satisfying. It is commonly believed that the research and development path of the treatment is full of obstacles and high-profiles failures. Many pharmaceutical companies around the world failed to stick to the last.
According to Reuters, a well-known U.S. company in this field Biogen renewed their plans for the treatment—aducanumab last week, after they had decided to abandon the two late-stage trials in March when a so-called "futility analysis" revealed the trials had little hope of succeeding.
But on Tuesday, Biogen said more data had become available after the two studies were discontinued, resulting in new analysis that showed one of the trials met the main goal. "They (FDA) thought it was reasonable for us to submit an application for approval," Alfred Sandrock, the company's R&D head told Reuters. And a sale application submitted by Biogen is said to be ready in early 2020. However, Some analysts said that it's not an easy task since they have to convince regulators the results are not from random chance before they get approval.
(Hu Nan also contributed to the story.)