The clinical laboratory medical center of the 5th medical center of the Chinese People's Liberation Army (PLA) General Hospital has been approved by the Logistics Support Department of the PLA Military Commission and the Beijing Municipal Health Commission, becoming the first laboratory for COVID-19 diagnosis and confirmation in the PLA.
"After becoming a laboratory for diagnosis, our test results can be reported directly without sending them to other units for review, which will greatly shorten the waiting time for diagnosis and treatment of patients, and reducing the chance of suspected nosocomial infections", director Li Boan of the 5th medical center clinical laboratory medical center said.
02:27
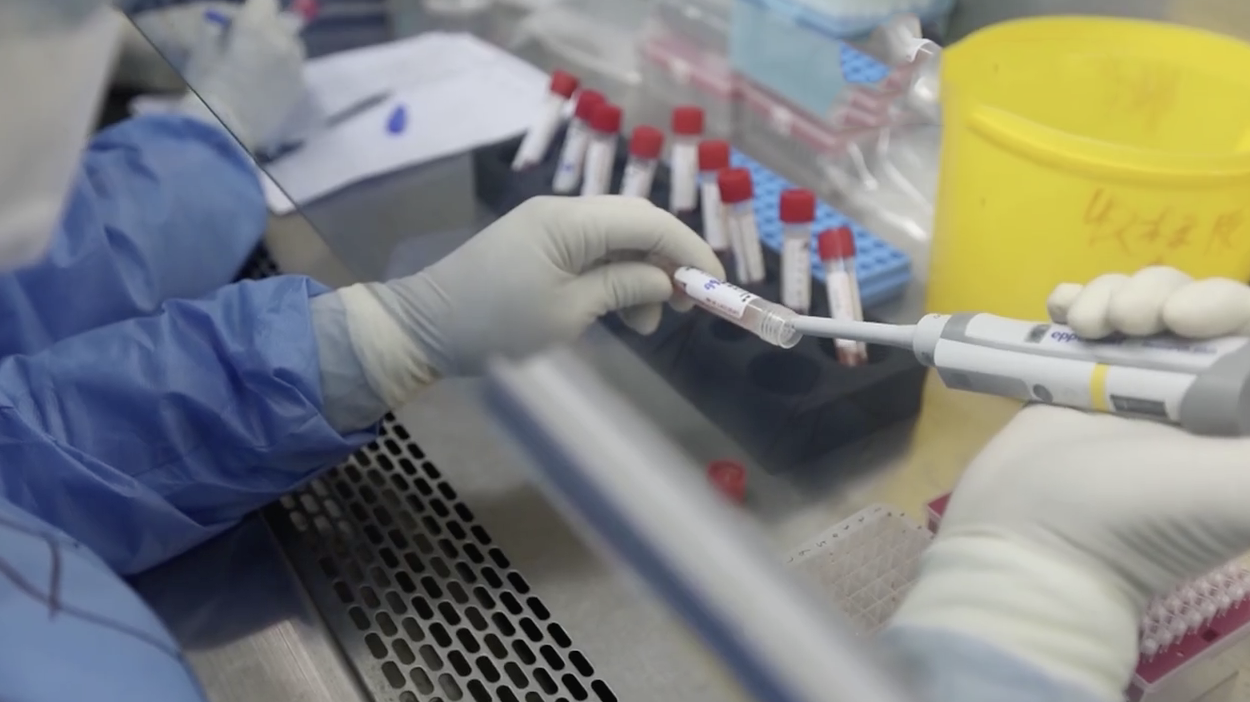
The test kit is the most prominent tool to test for the new coronavirus. It is not only responsible for screening suspect patients and determining whether confirmed patients can be discharged from hospitals, but also provides laboratory indicators and accurate diagnostic information.
Samples collected from suspect cases often came in bulk. After having samples disinfected, researchers need to extract the genetic material at a fast speed, then compare it with the known genetic sequence of the new coronavirus to find out whether they are identical.
To further improve the test speed and accuracy, the clinical lab center has developed a method that can increase the concentration of extract by 2.5 times.
According to the clinical center, the unannounced inspection result suggests the accuracy rate reached 100%.