Global research work on the novel coronavirus ranges from identifying the virus to modes of transmission and to treatment. The studies in those fields have all made breakthroughs.
At the early stage, scientists were focused on finding out where the virus (SARS-CoV-2) came from and how it was transmitted to humans.
Now the immediate priorities of research are focused on quickly identifying sick people and treating infected patients in order to curtail this outbreak as the global confirmed cases keep increasing.
What is the novel coronavirus and where is it from?
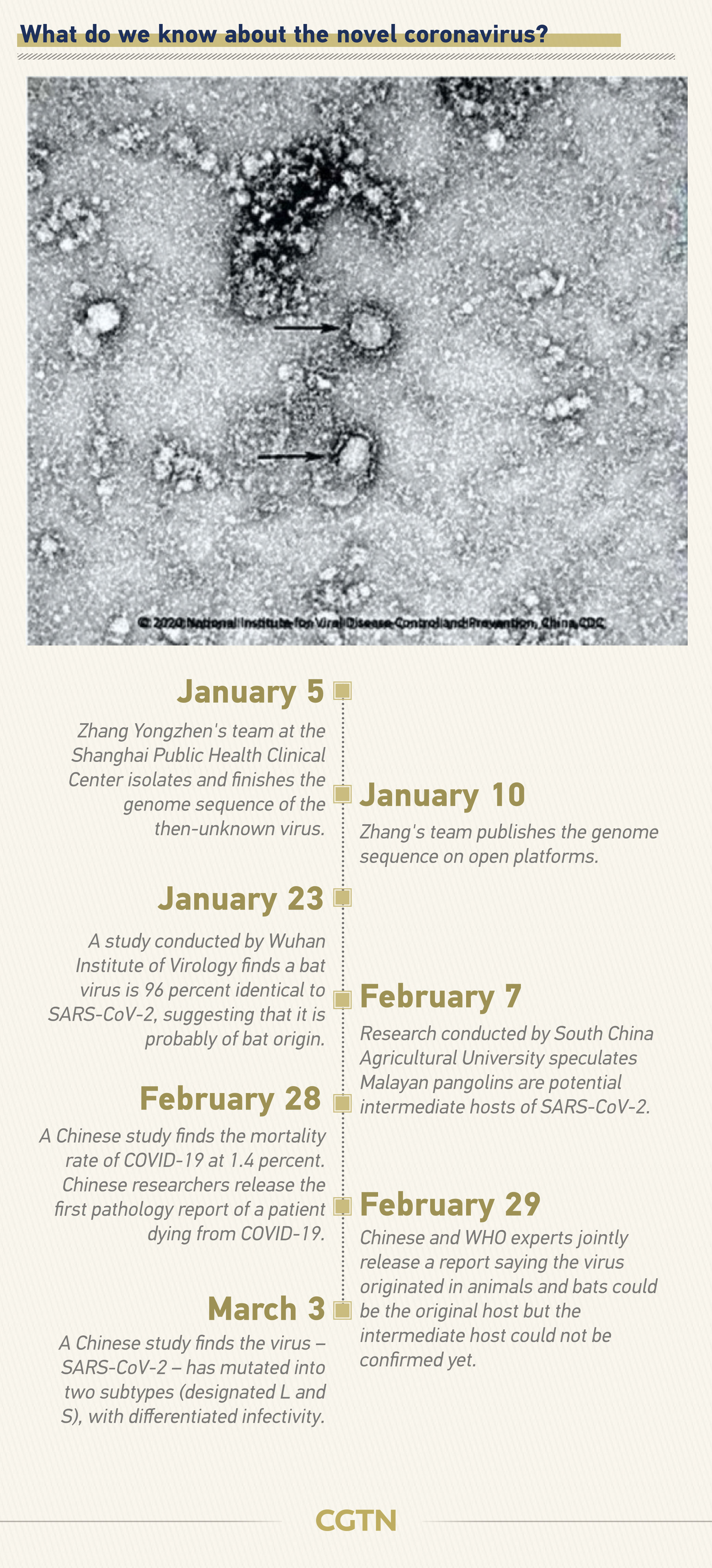
How is it transmitted?
SARS-CoV-2 is likely to be a coronavirus of bat origin, exhibiting 96.2 percent full genome identity with a clade 2b β-CoV from Rhinolophus affinis bats in Yunnan, China.
But the route of the spillover from animals to humans remains unclear. Potential candidates have been proposed for COVID-19, based on genomic similarities with related coronaviruses they host, for example, pangolins, at least for part of their genome.
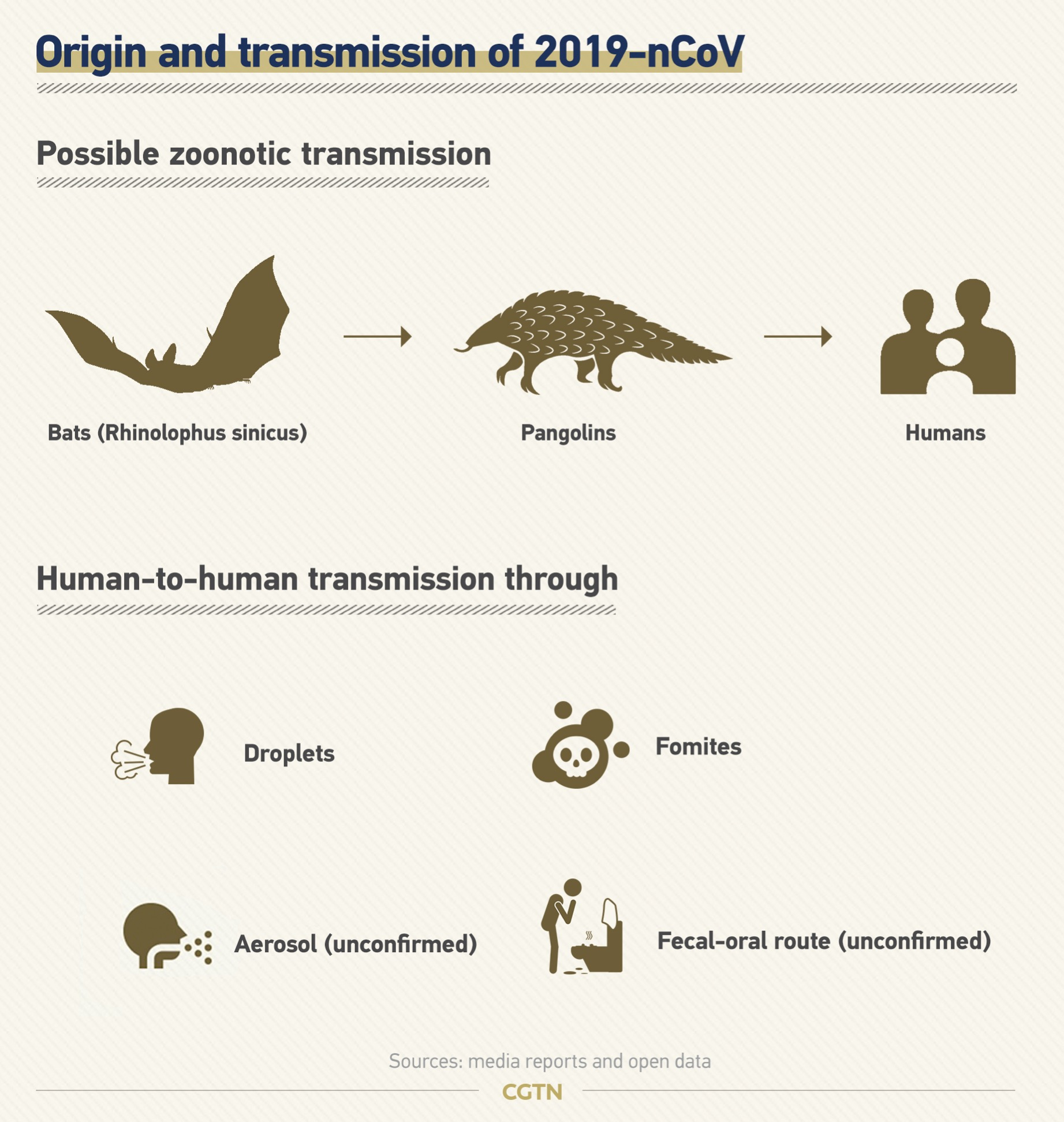
The main route of human-to-human transmission is respiratory droplets and close contact.
And there is the possibility of aerosol transmission when exposed to high concentration aerosol for a long time in a relatively closed environment.
Attention is also be paid to aerosol or contact transmission from feces and urine as new coronaviruses have been isolated here.
Regarding the latest findings on modes of transmission, Chinese researchers found that the novel coronavirus can be found in tears and conjunctive secretions, according to a study published on February 26.
Furthermore, asymptomatic transmission complicates scientific efforts to detect cases and to curb transmission.
A study published on February 19 indicates the asymptomatic infected carry the virus in similar to infected people with mild symptoms, after tracking and analyzing the viral load of 18 patients in Zhuhai City, south China's Guangdong Province.
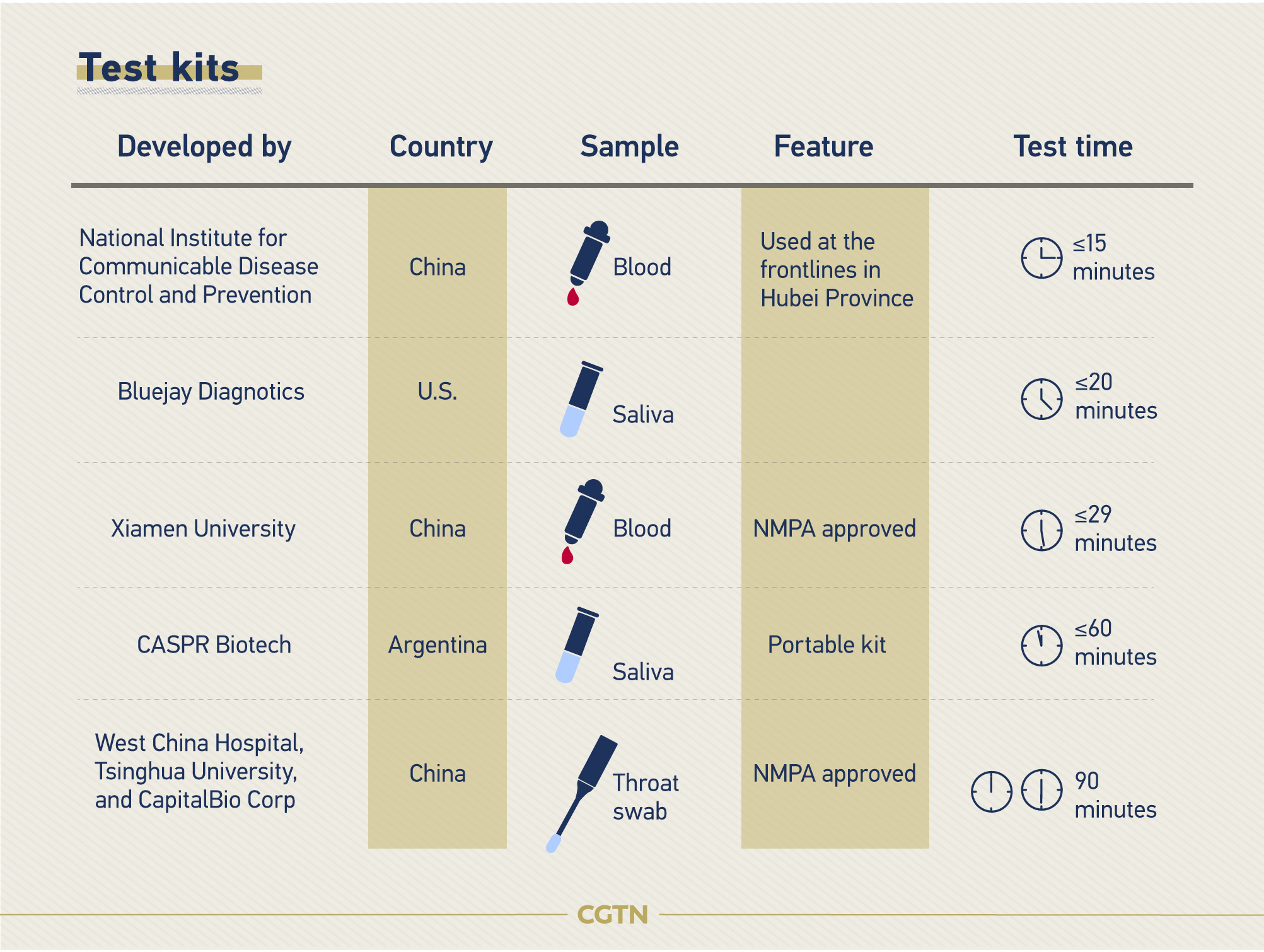
How is the novel coronavirus being tested?
The diagnosis of 2019-nCoV has been dependent on the positive result in real-time fluorescence RT-PCR detection of novel coronavirus nucleic acid. The shortage and long test time has pushed global researchers to develop more accurate and efficient test kits.
In response to the outbreak, China's National Medical Products Administration (NMPA) has adopted fast-track review and approval procedure for COVID-19 diagnostic kits.
Here are some of the newly developed test kits.
How is the COVID-19 being treated?
As of Saturday, 66,911 people in China have been cured and discharged.
The general treatment method is making the most of existing medicine and is targeted at controlling early symptoms.
The R&D on new coronavirus treatment mainly covers the trials of existing medicine and vaccine development.
There are currently no proven therapies or vaccines for COVID-19, but dozens of potential options are under clinical trial and more than 30 vaccines have been developed and are in the pre-clinical trial stage.
A number of large-scale randomized trials are being planned and are ongoing both inside and outside of China. Epidemiological studies, as conducted by public health authorities, have taken place in the U.S., Europe and other regions.
As of Saturday, 414 relevant clinical trials were registered on the Chinese Clinical Trial Registry, and 392 trials registered on the International Clinical Trials Registry Platform.

Bodies working to develop a vaccine include the Chinese Center for Disease Control and Prevention, Inovio Pharmaceuticals and Moderna in the U.S., University of Queensland in Australia, Serum Institute of India, University of Oxford in the UK and University of Saskatchewan in Canada.
It is important to strike the balance between curtailing the current epidemic now and preparing for the future.
The R&D of a vaccine from development to the market usually takes years. For example, it took more than two decades of research before the world finally got an approved Ebola vaccine.
How has an outbreak changed the communication and sharing among researchers?
The intense communication and information sharing among researchers is unprecedented and has resulted in a level of collaboration among scientists that has led to research actions to be implemented faster than ever before during the outbreak.
Once the Chinese researchers published the genome sequence of the virus in January, analysis among global researchers started right away.
Viruses and reagents are being globally mapped out to facilitate the sharing of samples and sequences.
Many researchers have been publishing their COVID-19 work through "pre-print" services like bioRxiv, which publishes papers that haven't yet been peer reviewed in an effort to get potentially life-saving research into public hands more quickly, and platforms like genomic data sharing service GISAID, to optimize resources, avoid duplication and cover research priorities.
(Graphics by Li Yueyun, Jia Jieqiong, Qu Bo)