A malaria drug, widely touted as a potential cure for COVID-19, showed no benefits against the disease over standard care and was in fact associated with more deaths, the biggest study of its kind showed on Tuesday.
The U.S. government-funded analysis of how American military veterans fared on hydroxychloroquine was posted on a medical preprint site and has not yet been peer-reviewed.
Researchers looked at the medical records of 368 veterans hospitalized nationwide who either died or were discharged by April 11.
Death rates for patients on hydroxychloroquine were 28 percent, compared to 22 percent when it was taken with the anti-biotic azithromycin - a combination favored by French scientist Didier Raoult, whose study on the subject in March triggered a surge of global interest in the drug.
The death rate for those receiving only standard care was 11 percent.
Hydroxychloroquine, with or without azithromycin, was more likely to be prescribed to patients with more severe illness, but the authors found that increased mortality persisted even after they statistically adjusted for higher rates of use.
Other drawbacks include the fact that the study did not assign people randomly to groups, because it was a retrospective analysis meaning it looked back on what had already happened.
In addition, the results are hard to generalize because the population was highly specific: most of the patients were male, with a median age over 65, and black, a group that is disproportionately affected by underlying illnesses like diabetes and heart disease.
There was no added risk of being on a ventilator among the hydroxychloroquine only group, leading the authors to suggest that increased mortality among this group might be attributable to side-effects outside the respiratory system.
Previous research has found that this medicine is risky for patients with certain heart rhythm issues and can cause blackouts, seizures or, at times, cardiac arrest.

Chinese top respiratory expert Zhong Nanshan shares his clinical trial results with global experts /CGTN
Chinese top respiratory expert Zhong Nanshan shares his clinical trial results with global experts /CGTN
The malaria drug in the U.S.
On April 8, the U.S. Centers for Disease Control and Prevention has removed from its website the highly unusual guidance informing doctors on how to prescribe hydroxychloroquine and chloroquine, drugs, both aggressively promoted by U.S. President Donald Trump to treat the coronavirus.
The move came three days after Reuters reported that the CDC published key dosing information involving the two anti-malaria drugs based on unattributed anecdotes rather than peer-reviewed science.
According to the initial CDC webpage, titled "Information for Clinicians on Therapeutic Options for Patients with COVID-19": "Although optimal dosing and duration of hydroxychloroquine for treatment of COVID-19 are unknown, some U.S. clinicians have reported anecdotally" on several ways to prescribe the medication of COVID-19.
The original guidance was crafted by the CDC after U.S. President Trump personally pressed federal regulatory and health officials to make the malaria drugs more widely available to treat the novel coronavirus diseases, though the drugs in question had been untested for COVID-19.
Chinese top respiratory expert Zhong Nanshan shares his clinical trial results with global experts /CGTN
Chinese top respiratory expert Zhong Nanshan shares his clinical trial results with global experts /CGTN
Opposite results in China
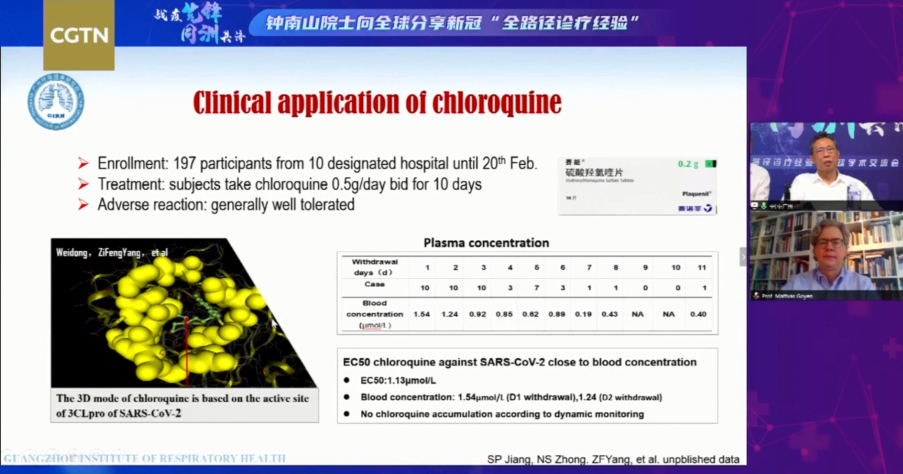
Just one week ago, Chinese top respiratory expert Zhong Nanshan told in a global webinar with tens of thousands of medical workers based in Europe, U.S., and Africa that new results in their clinical trials show the viral load in the plasma of patients treated with chloroquine has seen a rapid decrease.
And for the control group who were given both chloroquine and azithromycin, an antibiotic, the results are even more promising.
Previous clinical evidence from over 10 hospitals in Beijing, Guangzhou and Hunan Province also show the cure rate of patients who are negative in nucleic acid tests after treatment with the antimalarial drug is higher than in the controlled group. Over 100 patients have shown no signs of side effects, the expert said.
Hydroxychloroquine and related compound chloroquine have been used for decades to treat malaria, as well as the autoimmune disorders lupus and rheumatoid arthritis.
They have received significant attention during the novel coronavirus pandemic and have been shown in lab settings to block the virus from entering cells and prevent it replicating - but in the pharmaceutical world, "in vitro" promise often fails to translate into "in vivo" success.
The true answer can only be determined through very large, randomized clinical trials that assign patients to receive either the drug under investigation or a placebo.