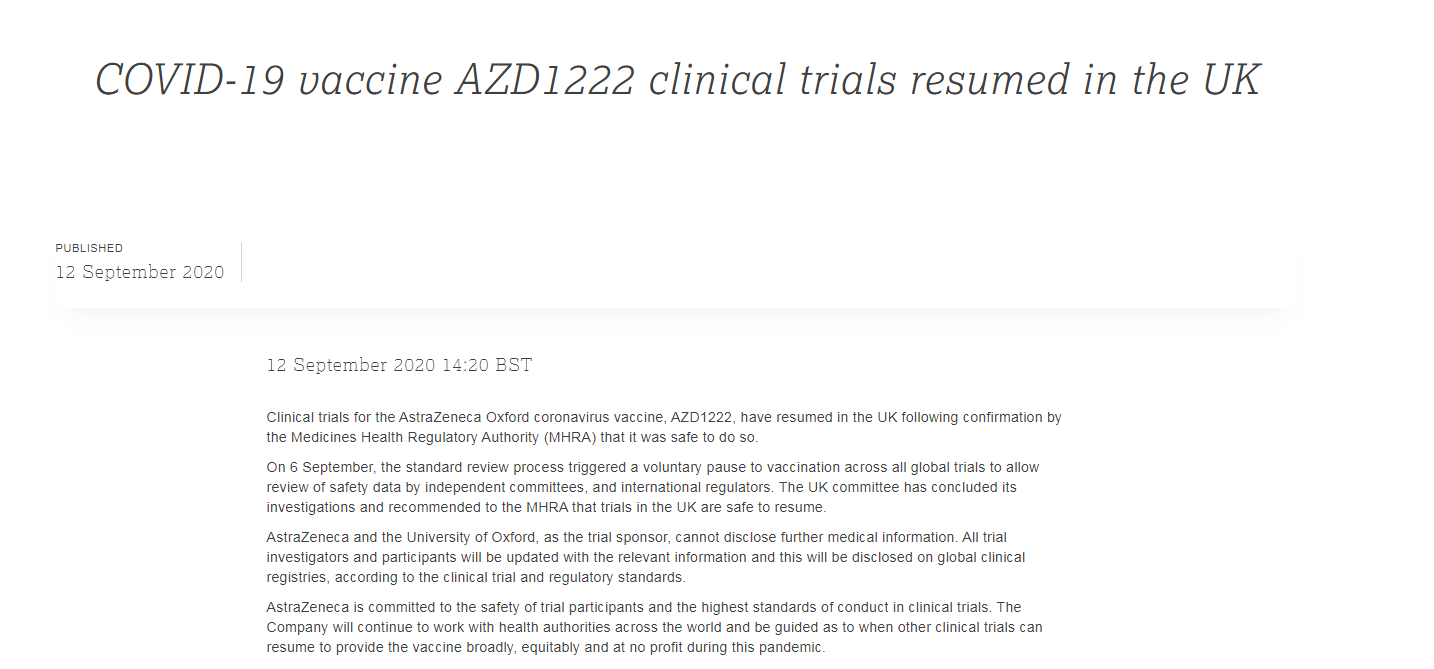
Clinical trials of the AstraZeneca and Oxford University coronavirus vaccine have resumed following confirmation by the Medicines Health Regulatory Authority (MHRA) that it was safe to do so, the pharmaceutical company and university said on Saturday.
"The UK committee has concluded its investigations and recommended to the MHRA that trials in the UK are safe to resume," AstraZeneca said. "The ongoing randomized controlled clinical trials of the Oxford coronavirus vaccine ChAdOx1 nCoV-19 will resume across all UK clinical trial sites," the Oxford University added.
On Wednesday, clinical trials conducted by AstraZeneca and Oxford University were put on temporary hold after a patient suffered a suspected serious side effect.
Screenshot from AstraZeneca's website
Screenshot from AstraZeneca's website
An AstraZeneca spokesperson confirmed the pause in vaccinations covered studies in the U.S. and other countries.
Late last month, AstraZeneca began recruiting 30,000 people in the U.S. for its largest study of the vaccine. It is also testing the vaccine in thousands of people in Britain, and in smaller studies in Brazil and South Africa.
Read more:
Clinical trials on UK's COVID-19 vaccine paused after one illness