01:11
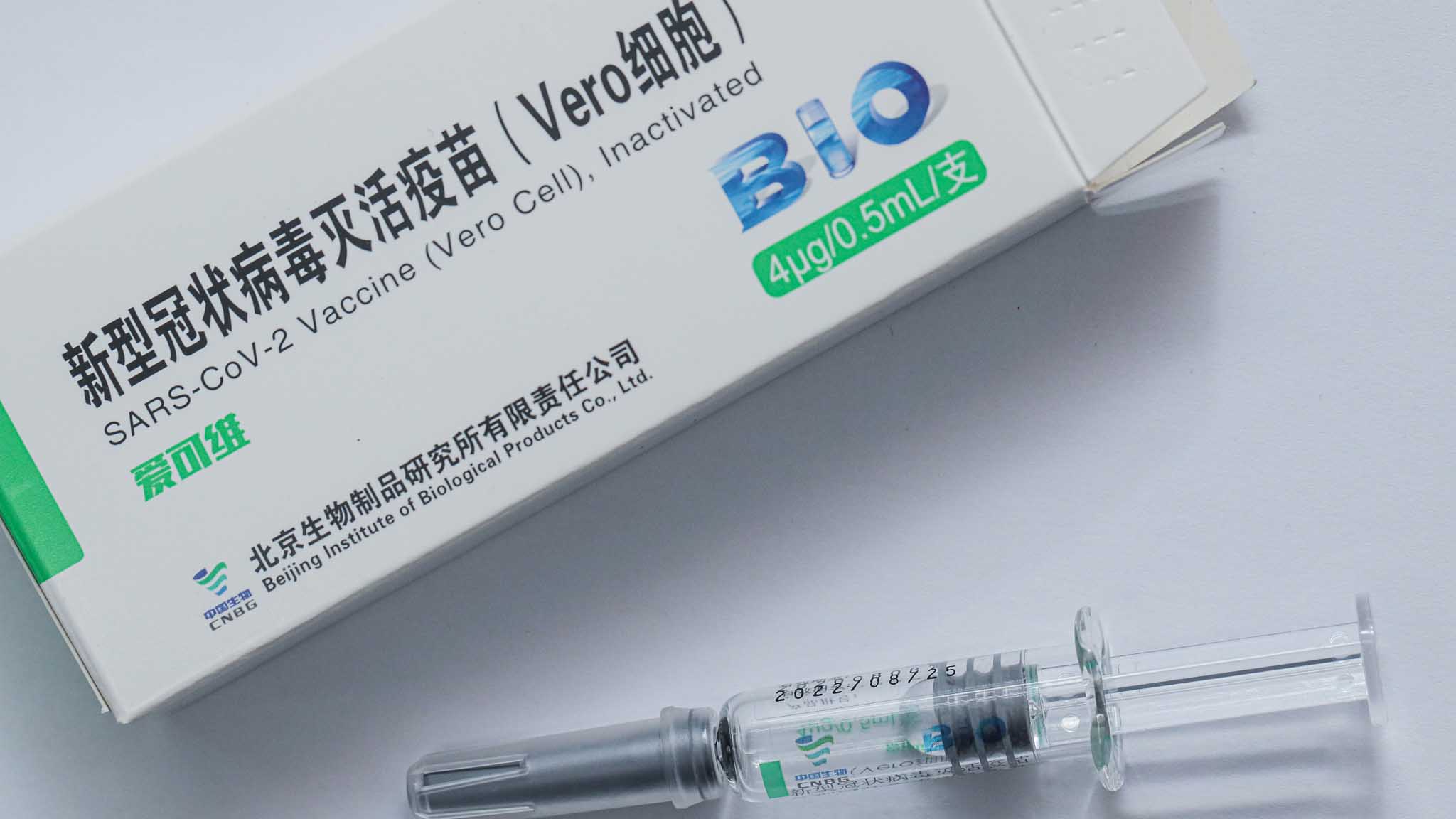
The China National Pharmaceutical Group Co., Ltd. (Sinopharm) announced on Saturday that its COVID-19 vaccine has acquired the GMP certificate issued by the Hungarian National Institute of Pharmacy and Nutrition (OGYEI), becoming the first Chinese COVID-19 vaccine to receive such a certificate.
With a Chinese COVID-19 vaccine receiving the first Good Manufacturing Practice (GMP) certificate issued by the Hungarian authorities, Chinese jabs are reportedly a step closer to becoming a global public good. At least 100 million doses of COVID-19 vaccines from Sinopharm have been supplied around the world.

