
People walk on the banks of the Ganges river during Kumbh Mela in Haridwar, India, April 12, 2021. /Reuters
People walk on the banks of the Ganges river during Kumbh Mela in Haridwar, India, April 12, 2021. /Reuters
India on Tuesday said it will fast-track emergency approvals for COVID-19 vaccines authorized by Western countries and Japan after the country witnessed the world's biggest surge in cases this month.
Soaring growth
Since April 2, India has reported the highest daily tallies of infections. It reported 161,736 cases on Tuesday, taking the total to 13.7 million, while deaths rose by 879 to 171,058.
Besides the ongoing religious gathering Kumbh Mela, the spike in cases is also attributed to mass election rallies by Prime Minister Narendra Modi's party and opposition groups during polls in four states and one federally run region.
The jump in India's infections, for which Health Minister Harsh Vardhan acknowledged a failure to heed curbs on movement and social interaction, has prompted calls for the government to cancel large public events.
On Tuesday, India's richest state Maharashtra, which accounts for about a quarter of the country's cases, said it would impose stringent restrictions from Wednesday to try to contain the spread.
It's reported that gatherings of more than four and nonessential travel on public transport would be banned for the next 15 days in Maharashtra.
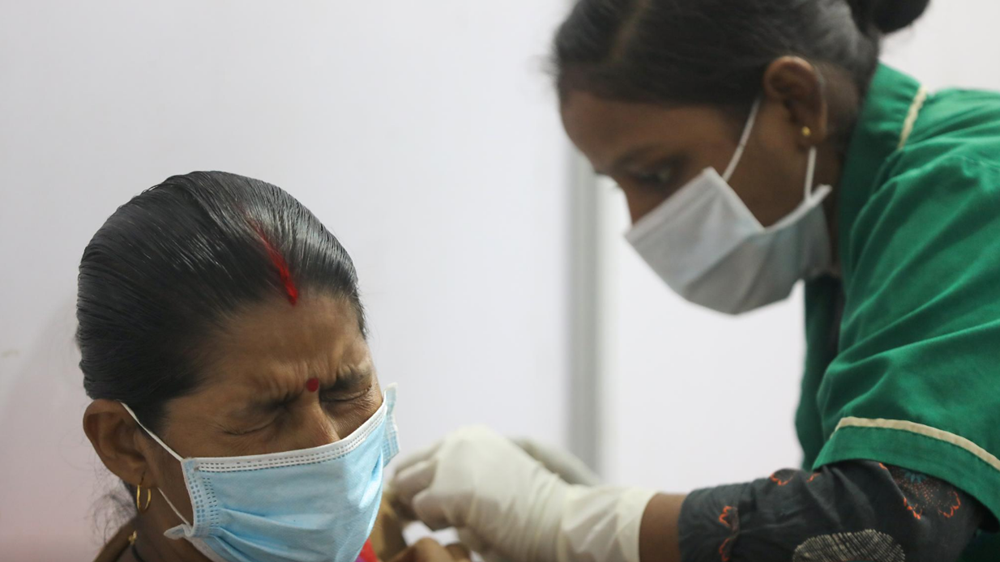
A woman receives a vaccine shot in Mumbai, India, April 11, 2021. /Reuters
A woman receives a vaccine shot in Mumbai, India, April 11, 2021. /Reuters
Vaccines imports granted
Home to the largest vaccine maker in the world, India had exported tens of millions of doses before its own demand skyrocketed and led to a shortage in some states.
The health ministry said vaccines authorized by the World Health Organization or authorities in the United States, Europe, Britain and Japan could be granted emergency use approval in India.
"If any of these regulators have approved a vaccine, the vaccine is now ready to be brought into the country for use, manufacture and fill-and-finish," said Vinod Kumar Paul, a senior government health official.
"We hope and we invite the vaccine makers such as Pfizer, Moderna, Johnson & Johnson and others to be ready to come to India as early as possible."
U.S. federal health agencies on Tuesday recommended pausing the use of the J&J shot after six women under age 50 developed rare blood clots after receiving it.
India is currently using the AstraZeneca shot and a homegrown vaccine for its immunization drive. This week, it approved Russia's Sputnik V shot for emergency use after it overtook Brazil to become the country with the second-highest number of cases globally.
(with input from Reuters)

