00:29

Editor's note: This is the weekly wrap-up of Chinese COVID-19 vaccines' contribution in the global fight against the virus for May 24-30 2021.
Two COVID-19 vaccines developed by China's Sinopharm have shown an efficacy of over 72 percent in large scale phase-3 clinical trials, according to a study published in The Journal of the American Medical Association (JAMA) on Wednesday.
The two inactivated vaccines, developed by Sinopharm's Wuhan Institute of Biological Products and Beijing Institute of Biological Products, showed an efficacy of 72.8 percent and 78.1 percent respectively against symptomatic COVID-19 cases, with rare serious adverse effects reported and it is the world's first published phase-3 study results of inactivated COVID-19 vaccines.
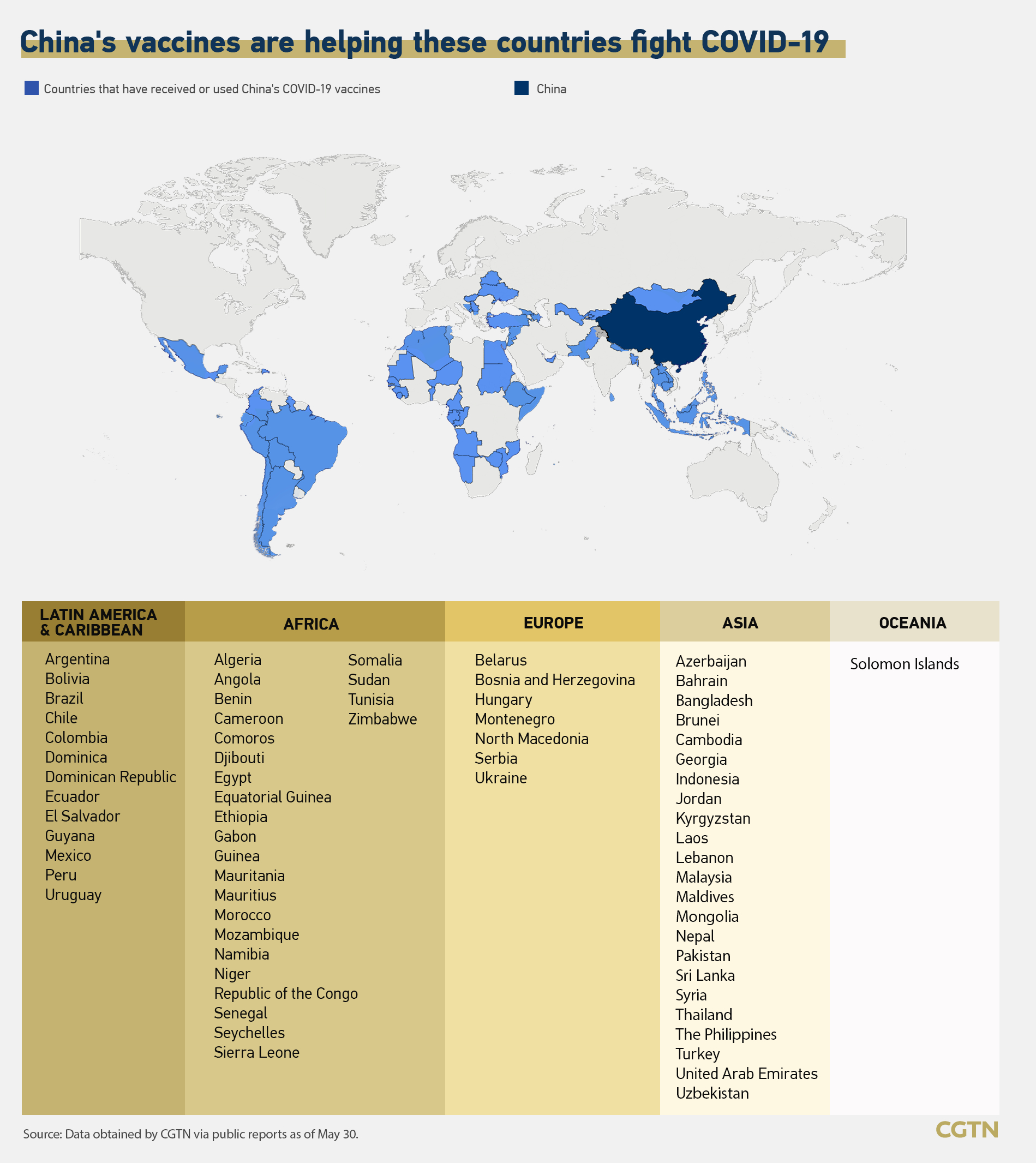
China promised an additional $3 billion in international aid over the next three years to support COVID-19 response and economic and social recovery in other developing countries at the Global Health Summit via video on May 21. Chinese vaccines are now part of the immunization campaigns in many developing countries, including in Africa nations, and have gained more recognition worldwide.
China's Sinovac Biotech Ltd. on Friday said it will set up a national center as part of the BRICS Vaccine Research and Development Center, boosting the vaccine research and development in Brazil, Russia, India, China, and South Africa (BRICS).
02:23
Egyptian health authorities on Friday received a shipment from China that includes raw bio-material from Sinovac, which will help Egypt begin producing the Chinese vaccine domestically, making it the first African nation to produce COVID-19 vaccines.
Thailand's food and drug regulator on Friday approved Sinopharm vaccines for emergency use, senior health official Paisan Dankhum told a news conference, making it the fifth COVID-19 vaccine Thailand has approved.
Also on Friday, around 700 Philippine athletes bound for the Tokyo Olympics and Southeast Asian Games received the Sinovac coronavirus vaccine.
An "important call at a fateful time"
Chile's President Sebastian Pinera on May 23 welcomed a shipment of 2.2 million COVID-19 vaccine doses made by Chinese pharmaceutical firm Sinovac, the largest batch of vaccine doses the country has ever received, according to local media.
Addressing the press at a briefing at Santiago International Airport upon the batch's arrival, the president said 7.6 million people in Chile have received both doses and 9.5 million have received at least one shot.
The president himself has received two Sinovac shots, taken in February and March, respectively.
Libyan experts said that China's recent appeal for the international community to urgently provide COVID-19 vaccines to Africa is "an important call at a fateful time," a Xinhua report said on Tuesday.

"Beijing, through its call on the United Nations Security Council (UNSC) to provide vaccines, is keen to mobilize international support for African countries, most of which suffer from poverty and economic problems," said Khaled Al-Tarhouni, a Libyan political analyst.
He believes China will have a great and influential role in providing vaccines to Africa, especially after its recent agreement with Egypt to establish a Chinese vaccine production line in Egypt.
Serious adverse effects "extremely low"
The rate of serious adverse effects from China's COVID-19 vaccines is extremely low, and the benefits of vaccination far outweigh the risks, the Chinese Center for Disease Control and Prevention (CDC) said on Friday.
According to CDC statistics, 31,434 adverse reaction cases were reported on the Chinese mainland between December 15, 2020, and April 30, 2021, after 265 million COVID-19 vaccine doses were administered, with a rate of 11.86 per 100,000 doses.
Fever and swelling accounted for 82.96 percent or 9.84 per 100,000 doses in normal reactions. Meanwhile, abnormal reactions like an allergic rash accounted for 17.04 percent or 2.02 per 100,000 doses. A total of 188 cases of severe adverse reactions were reported, with the incidence of 0.07 per 100,000 doses, which means "extremely rare," the CDC said.

