00:29
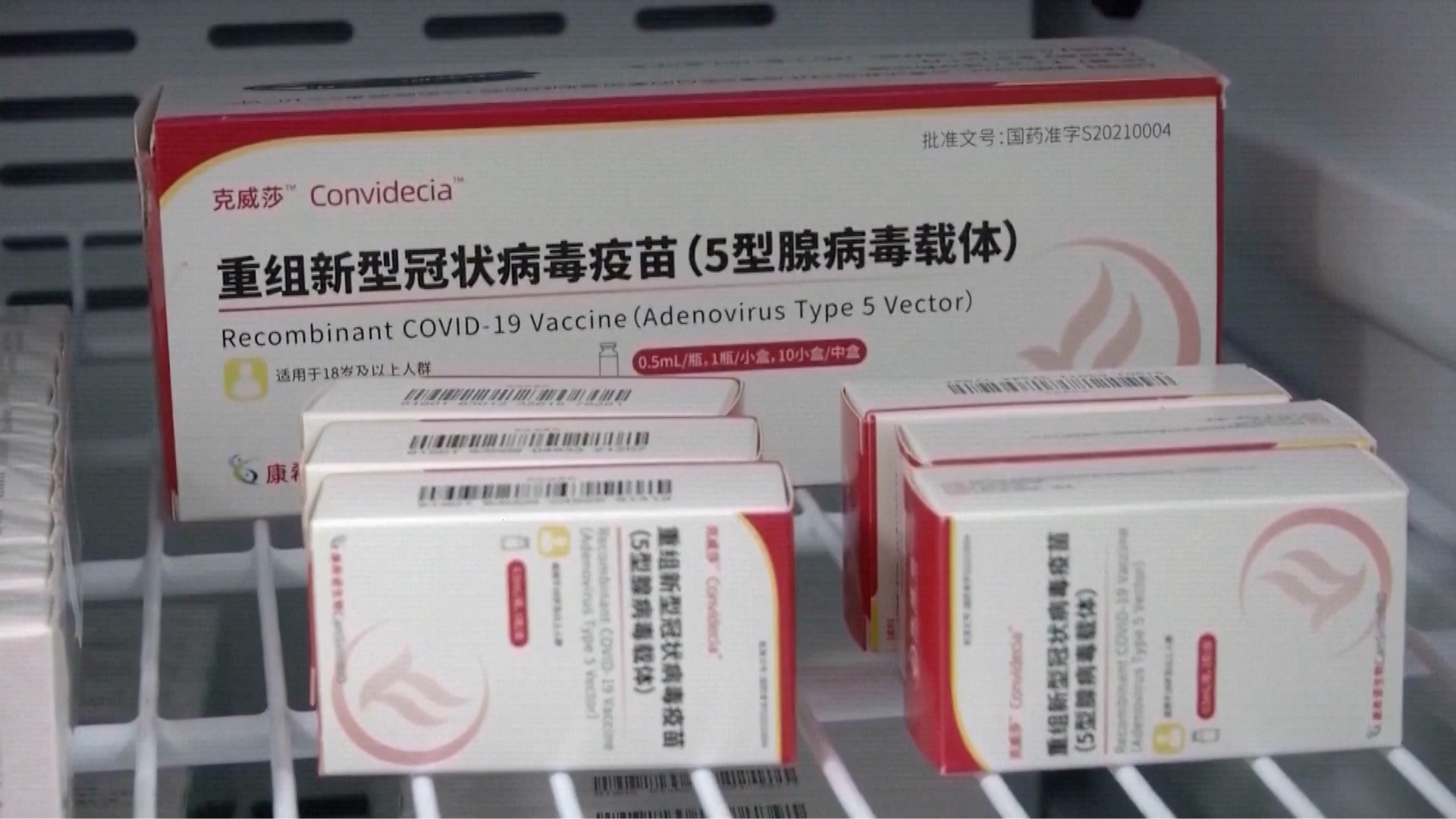
On May 19, the World Health Organization (WHO) has validated the CONVIDECIA COVID-19 vaccine developed by Chinese developer CanSino Biologics for emergency use. CONVIDECIA is the third Chinese vaccine to be recognized by the WHO after Sinopharm and Sinovac on May 7 and June 1, 2021 respectively. The 11th vaccine validated by the WHO for COVID-19 prevention, CONVIDECIA is a COVID-19 adenoviral vector vaccine that is suitable for adults.

