
Tech & Sci
11:36, 09-Jul-2017
Stanford uses copper to produce corn-free ethanol

A recent discovery by a team of Stanford University researchers could lead to a new, more sustainable way of making ethanol without corn or other crops, potentially producing renewable energy without affecting food sources.
The technology has three basic components, namely water, carbon dioxide and electricity delivered through a copper catalyst.
It is expected to address the status quo that most cars and trucks in the United States run on a blend of 90 percent gasoline and 10 percent ethanol, a renewable fuel made primarily from fermented corn. Some 53 billion liters of ethanol are consumed annually by American drivers, requiring millions of acres of farmland.
"One of our long-range goals is to produce renewable ethanol in a way that doesn't impact the global food supply," said Thomas Jaramillo, an associate professor of chemical engineering at Stanford and principal investigator of the study, which was published in Proceedings of the National Academy of Sciences.

Associate Professor Thomas Jaramillo (L) and SLAC scientist Christopher Hahn have demonstrated the feasibility of designing copper catalysts that convert carbon dioxide into ethanol without corn or other crops. /Stanford University Photo
Associate Professor Thomas Jaramillo (L) and SLAC scientist Christopher Hahn have demonstrated the feasibility of designing copper catalysts that convert carbon dioxide into ethanol without corn or other crops. /Stanford University Photo
"Copper is one of the few catalysts that can produce ethanol at room temperature," Jaramillo noted. "You just feed it electricity, water and carbon dioxide, and it makes ethanol."
"The problem is that it also makes 15 other compounds simultaneously, including lower-value products like methane and carbon monoxide. Separating those products would be an expensive process and require a lot of energy," he added.
Therefore, the goal is to design copper catalysts that selectively convert carbon dioxide into higher-value chemicals and fuels, like ethanol and propanol, with few or no byproducts.
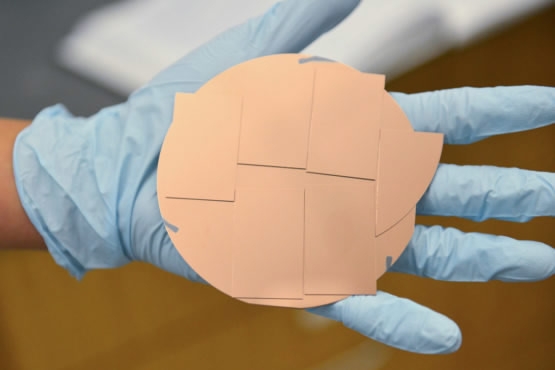
Scientists have designed a large copper catalyst that produces ethanol from carbon dioxide and water. /Stanford University Photo
Scientists have designed a large copper catalyst that produces ethanol from carbon dioxide and water. /Stanford University Photo
Ultimately, the Stanford team would like to develop a technology capable of selectively producing carbon-neutral fuels and chemicals at an industrial scale.
"The eye on the prize is to create better catalysts that have game-changing potential by taking carbon dioxide as a feedstock and converting it into much more valuable products using renewable electricity or sunlight directly," Jaramillo said.

SLAC scientist Christopher Hahn sees his reflection in a shiny copper catalyst that converts carbon dioxide into ethanol. /Stanford University Photo
SLAC scientist Christopher Hahn sees his reflection in a shiny copper catalyst that converts carbon dioxide into ethanol. /Stanford University Photo
There have long been debates over whether ethanol is more costly than gasoline as its production depends on a large consumption of corn, and roughly 40 percent of US corn today is used for biofuel production.
Animal feed also counts for roughly 36 percent of US corn, plus distillers' grains leftover from ethanol production that is fed to cattle, pigs and chickens. Much of the rest is exported.
It takes more than 200 kilograms of corn to produce enough ethanol to fill one SUV tank. That’s enough corn to feed one person for a year. People argue that as crops, which were previously meant for food, are now being used to make ethanol, the world population suffering from poverty and hunger is greatly affected.
This new discovery sheds light on the development of renewable energy, without having to make compromises on food sources.
(With inputs from Xinhua)
9554km
Related stories:

SITEMAP
Copyright © 2018 CGTN. Beijing ICP prepared NO.16065310-3
Copyright © 2018 CGTN. Beijing ICP prepared NO.16065310-3