
Health
11:02, 23-Nov-2017
HPV vaccine developed in China gets permission for clinical testing
By Wang Xueying
The second-generation human papilloma virus (HPV) vaccine developed in China has been officially approved by the China Food and Drug Administration (CFDA) for clinical testing, according to multiple Chinese media sources on Wednesday.
Following the one developed by Merck & Co. (MSD), Gardasil-9, the new vaccine is the world's second second-generation HPV vaccine, to get permission to start the clinical test stage in China. Compared with the first-generation vaccine, the new one can protect against five more high-risk types of HPV and two more low-risk types. It is estimated to be used to prevent about 90 percent of cervical cancers and genital warts.

Chinese researchers are developing new HPV vaccines./China Daily Photo
Chinese researchers are developing new HPV vaccines./China Daily Photo
The research is led by the National Institute of Diagnostics and Vaccine Development in Infectious Diseases, based at Xiamen University in southeast China's Fujian Province. According to the researchers, the new vaccine uses more cost-effective coliform bacteria as the effective antigen, while most foreign companies such as MSD prefer using yeast cells. It can largely reduce the manufacturing cost.
In the past, many women on the Chinese mainland preferred to fly to Hong Kong to get vaccinated against HPV. But things started to change in 2017.
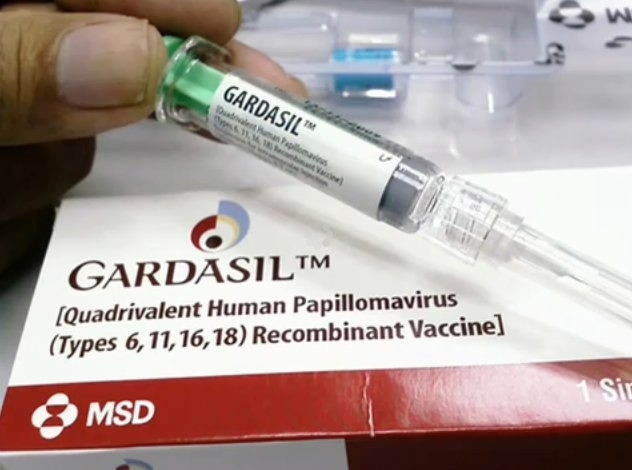
MSD’s Gardasil-9 was approved by China in May./AFP Photo
MSD’s Gardasil-9 was approved by China in May./AFP Photo
This July, Cervarix-2, an HPV vaccine manufactured by GlaxoSmithKline (GSK), got the permission from the CFDA to be sold on the Chinese mainland. It is the first first-generation HPV vaccine approved by the CFDA and is expected to be commercially available by the end of 2017.
At the same time, MSD’s Gardasil-9 was approved by China in May, becoming the first foreign second-generation HPV vaccine licensed for use on the Chinese mainland. The first patient has already vaccinated in southwest China's Chongqing Municipality on Nov. 13.
If everything goes as plan, the new vaccine will be put into production by 2022. /VCG Photo
If everything goes as plan, the new vaccine will be put into production by 2022. /VCG Photo
Additionally, Alibaba has announced it will cooperate with GSK to provide users with online consultation services. People could book appointments for HPV vaccinations in more than 100 cities in China, reported Chinese media outlets in November.
If everything goes as plan, the new second-generation HPV vaccine developed by China will be put into production in Xiamen by 2022.
Source(s): Global Times

SITEMAP
Copyright © 2018 CGTN. Beijing ICP prepared NO.16065310-3
Copyright © 2018 CGTN. Beijing ICP prepared NO.16065310-3