
Tech & Sci
09:23, 02-Jul-2018
US scientists develop inexpensive catalyst to split water
Updated
09:02, 05-Jul-2018
CGTN
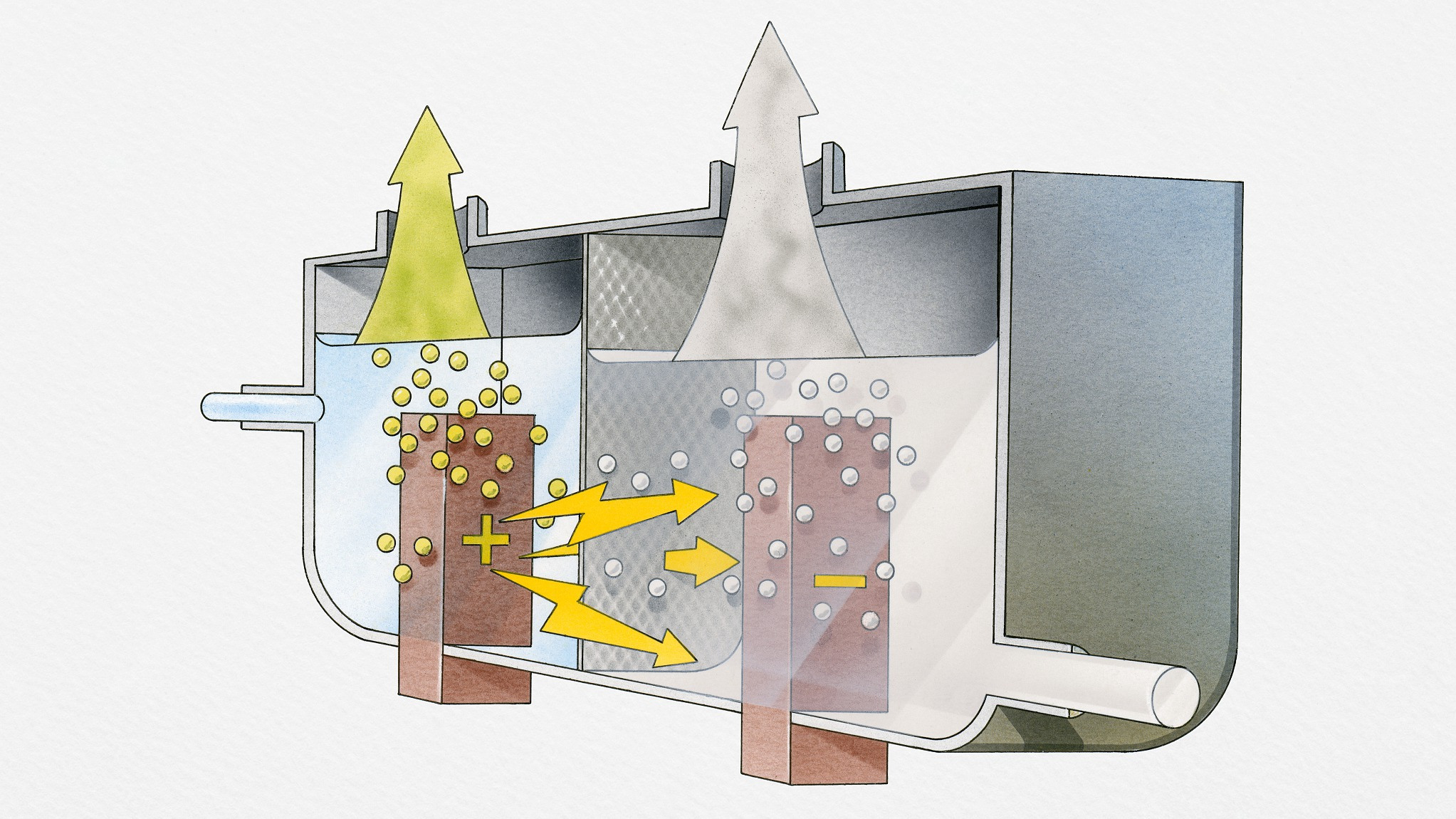
American researchers have developed an inexpensive hybrid catalyst capable of splitting water to produce hydrogen that has the potential for large-scale commercialization.
A recent study published in the journal Nature Communication described the new catalyst made of iron and dinickel phosphides (Ni3P2) on commercially available nickel foam.
Most systems to split water into hydrogen and oxygen require two catalysts: one to spur a reaction to separate the hydrogen; the other to produce oxygen.
But the new catalyst can perform both functions, according to researchers from the University of Houston (UH) and the California Institute of Technology.
Researchers said it has the potential to dramatically lower the amount of energy required to produce hydrogen from water while generating a high current density, a measure of hydrogen production.
"It puts us closer to commercialization," said Ren Zhifeng, professor of physics at UH and lead author of the paper.
The new catalyst is a bifunctional catalyst for overall water splitting, exhibiting both extremely high OER (oxygen evolution reaction) and HER (hydrogen evolution reaction) activities in the same alkaline electrolyte, according to the study.
Ren said the interaction between the iron phosphide particles and the dinickel phosphide particles boosted both reactions.
Hydrogen is considered a desirable source of clean energy, in the form of fuel cells to power electric motors or burned in internal combustion engines, along with a number of industrial uses.
While traditional catalysts can produce hydrogen from water, they generally rely on expensive platinum group elements. That raises the cost, making large-scale water splitting impractical, according to the researchers.
"In contrast, our materials are based on earth-abundant elements and exhibit comparable performance with those of platinum group materials," said Chen Shuo, assistant professor of physics at UH, the paper's co-author.
"It can be potentially scaled-up at low cost, which makes it very attractive and promising for the commercialization of water splitting," said Chen.
Researchers said the catalyst remained stable and effective through more than 40 hours of testing.
(Top image: VCG Photo)
Source(s): Xinhua News Agency

SITEMAP
Copyright © 2018 CGTN. Beijing ICP prepared NO.16065310-3
Copyright © 2018 CGTN. Beijing ICP prepared NO.16065310-3