
China
20:03, 22-Jul-2018
China gives update on vaccine scandal investigation
Updated
20:03, 25-Jul-2018
CGTN
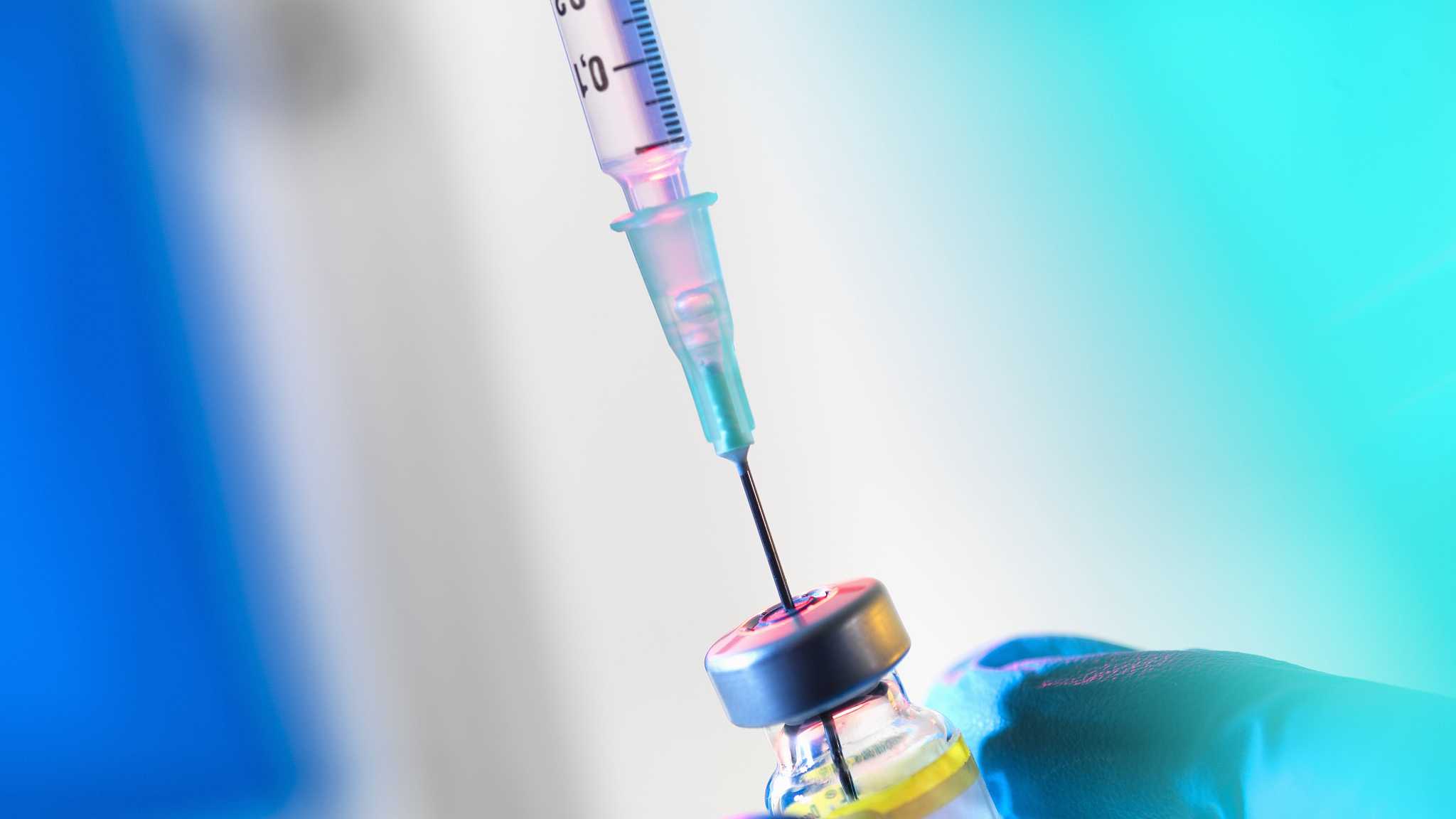
The National Drug Administration of China (CNDA) on Sunday provided an update on the investigation in the case of Changchun Changsheng Life Sciences, saying the company has been urged to stop production.
Authorities have launched a full investigation in this case, said Xu Jinghe, Deputy Commissioner of the CNDA.
According to the investigation, the company counterfeited the vaccine’s production data and changed the parameters for equipment and processing the vaccine, an act that violates good pharmaceutical manufacturing practices, said Xu.
More:

SITEMAP
Copyright © 2018 CGTN. Beijing ICP prepared NO.16065310-3
Copyright © 2018 CGTN. Beijing ICP prepared NO.16065310-3