
Tech & Sci
14:39, 24-Jul-2018
New malaria drug gets approval
Updated
14:04, 27-Jul-2018
Alok Gupta
A new drug to prevent recurring malaria has been approved by the US Food and Drug Administration (FDA). The simpler cure for the mosquito-borne disease has received a go-ahead after 60 years.
“Approval of Krintafel (tafenoquine), the first new treatment for Plasmodium vivax malaria in over 60 years, is a significant milestone for people living with this type of relapsing malaria,” Dr. Hal Barron, chief scientific officer, GSK said.
The drug, tafenoquine, will treat Plasmodium vivax malaria that infects more than 8.5 million people every year in Africa, Asia, and Latin America. According to the World Health Organization (WHO), 216 million cases of malaria and nearly 445,000 malaria deaths were reported in 2016.
Mosquitoes carrying the Plasmodium vivax parasites infect individuals with malaria. Even after treatment, the parasite remains dormant in the liver of the patients. These parasites flare up weeks, and sometimes years after the initial infection.
This recurring malaria is prevalent in countries outside African countries, creating a considerable burden on the public health system. However, primaquine, a drug administered over a 14-day period is available to cure dormant malaria. The extended period of the medication often results in relapses, especially in developing countries.
Developed by UK pharmaceutical giant GSK and Medicines for Malaria Venture (MMV), a non-profit, Tafenoquine, a single-dose treatment for malaria is being termed as a “major milestone" for malaria eradication.
“Today, we can say the wait is over. Moreover, as the first ever single-dose for this indication, tafenoquine will help improve patient compliance,” Dr. David Reddy, chief executive officer, MMV maintained.
“We are proud to have worked side-by-side with GSK for more than a decade to reach this point. Our focus is now on working to ensure the medicine reaches the vulnerable patients that need it most.”
The drug has been approved for use in the US, but GSK is learned to have applied for a similar go ahead in Australia. FDA warned that the new single-dose malaria comes with a few side effects.
“Serious psychiatric adverse reactions have been observed in patients with a previous history of psychiatric conditions at doses higher than the approved dose,” said FDA's approval letter.
Symptoms of malaria include fever, chills, vomiting headache and body ache. If not appropriately treated, the disease can be fatal.
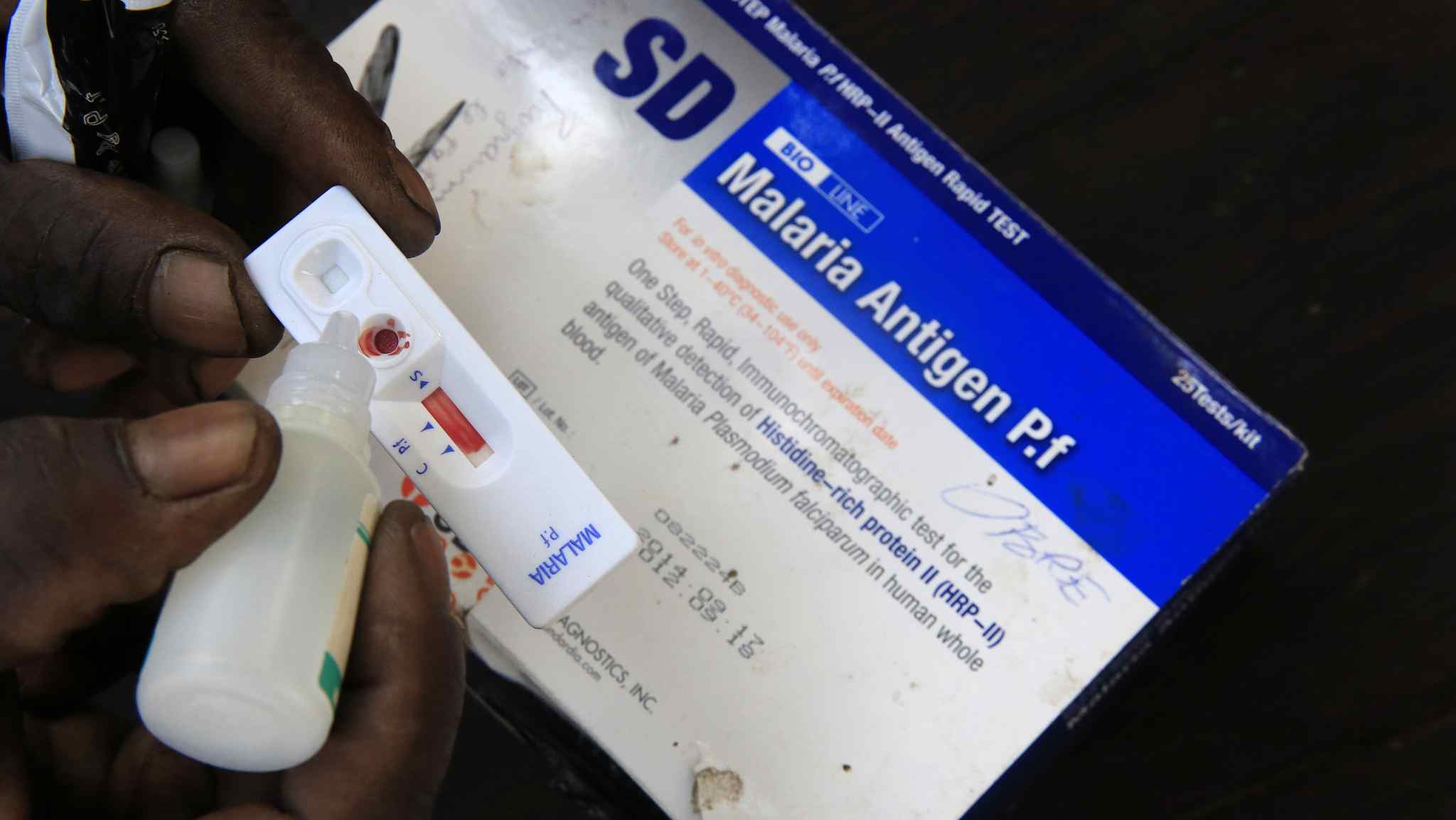
(Cover Image: A health worker testing a patient's blood for malaria using the rapid testing kit in an African village. /VCG Image)

SITEMAP
Copyright © 2018 CGTN. Beijing ICP prepared NO.16065310-3
Copyright © 2018 CGTN. Beijing ICP prepared NO.16065310-3