
Enviro
09:34, 12-Jun-2018
New material can capture toxic atmospheric gas multiple times
CGTN

An international team of scientists has developed a material that can remove nitrogen dioxide gas from the atmosphere in a selective, fully reversible and repeatable manner.
The discovery, published on Monday in the journal Nature Materials, was confirmed using neutron scattering technique at the United States Department of Energy's Oak Ridge National Laboratory.
According to the researchers, the metal-organic framework material (MOF) could lead to air filtration technologies that cost-effectively capture and convert large quantities of targeted gases, including carbon dioxide and other greenhouse gases, to facilitate their long-term sequestration to help mitigate air pollution and global warming.
It is capable of a reversible, selective capture of nitrogen dioxide at ambient pressures and temperatures, at low concentrations, in the presence of moisture, sulfur dioxide and carbon dioxide.
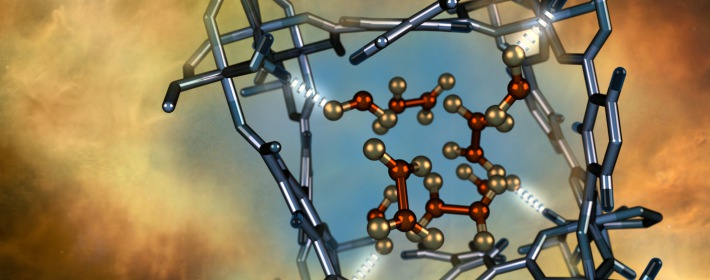
Illustration of a nitrogen dioxide molecule (depicted in red and gold) confined within a nano-sized pore of an MFM-300(Al) metal-organic framework material as characterized using neutron scattering at Oak Ridge National Laboratory. /Photo via Oak Ridge National Laboratory
Illustration of a nitrogen dioxide molecule (depicted in red and gold) confined within a nano-sized pore of an MFM-300(Al) metal-organic framework material as characterized using neutron scattering at Oak Ridge National Laboratory. /Photo via Oak Ridge National Laboratory
Despite the highly reactive nature of nitrogen dioxide, the material proved extremely robust, demonstrating the capability to be fully regenerated, or degassed, multiple times without loss of porosity.
The metal-organic framework material is a highly porous material, which is solid with many internal channels and holes. A sugar-cube-sized MOF might have an internal surface area the size of six football fields.
Capturing greenhouse and toxic gases from the atmosphere has long been a challenge, because of their relatively low concentrations and the presence of moisture in the air, which can negatively affect separating targeted gas molecules from other gases, according to the researchers.
Another challenge has been finding a practical way to release a captured gas for long-term sequestration, such as in underground depleted oil reservoirs or saline-filled rock formations.
The research team involved scientists from institutions in five nations, including the University of Manchester, the University of Nottingham, University of Newcastle upon Tyne, University of Nottingham Ningbo China, Peking University, the International Tomography Center SB RAS, Novosibirsk State University and the European Synchrotron Radiation Facility in Grenoble.
(Top image: VCG Photo)
Source(s): Xinhua News Agency

SITEMAP
Copyright © 2018 CGTN. Beijing ICP prepared NO.16065310-3
Copyright © 2018 CGTN. Beijing ICP prepared NO.16065310-3