By CGTN’s Wu Guoxiu
China's top food and drug authority conducted overseas inspections in 19 countries for imported pharmaceuticals last year. Officials found that some companies failed to meet Chinese drug standards. The China Food and Drug Administration's 2016 report released in Beijing on Wednesday covers its domestic and overseas drug inspections.
China inspected imported drugs from 19 countries last year, mostly in Europe and North America.
Of the 15 varieties of drugs inspected, three of them failed to pass, a higher rate than in previous years. Eight varieties withdrew their import registration certificates or had their applications returned during the inspections.
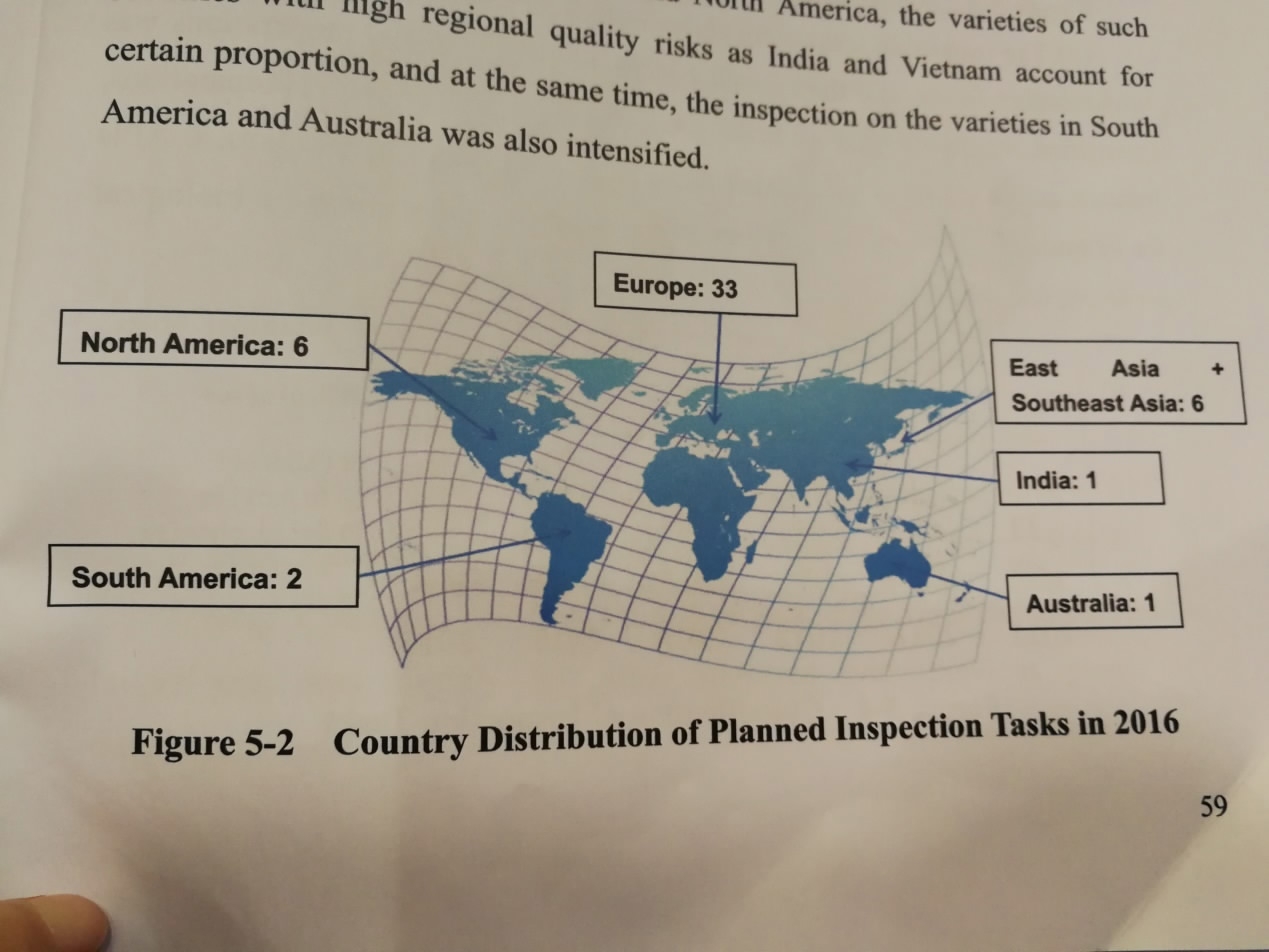
China Food and Drug Administration's 2016 report released in Beijing on May 31, 2017, presents country distribution of planned inspections to overseas drug companies by China in 2016. /CGTN photo
Ding Jianhua, director of Department of Drug & Cosmetics Registration of the CFDA says, "One manufacturer from a European Union country didn't report to the CFDA their major change and even added some APIs, active ingredients to their production, they didn't report their source of materials, we stopped the importation of them.”
Ding adds that foreign manufacturers do follow Chinese regulation. But they did find some small companies in some developing countries that don’t know Chinese regulations or forget about Chinese requirements.
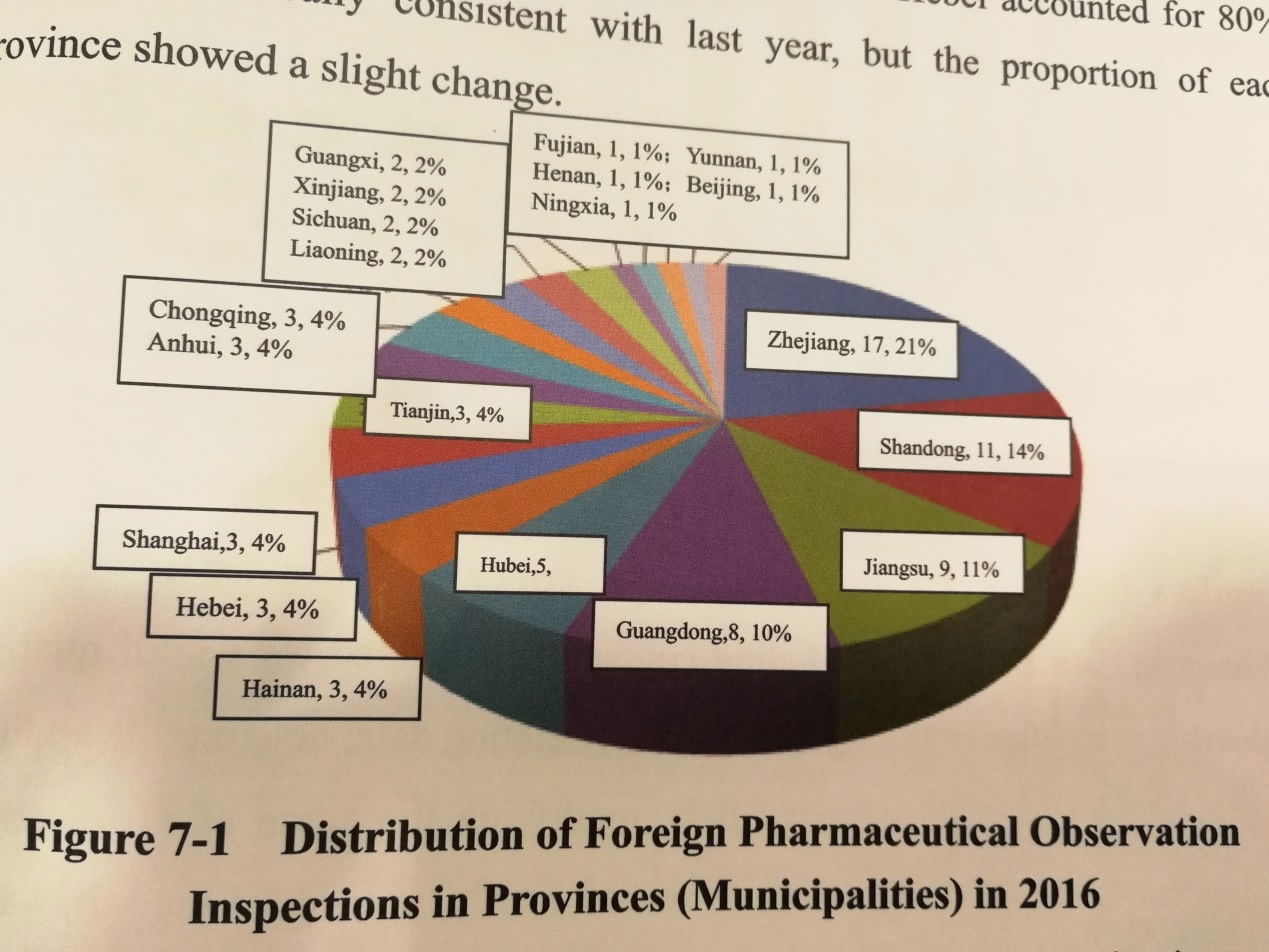
China Food and Drug Administration's 2016 report released in Beijing on May 31, 2017, presents distribution of Chinese drug companies inspected by foreign countries in 2016. /CGTN photo
It's an international practice for countries to inspect overseas drug-manufacturing plants to check on imported drugs. In 2016, China had at least 81 inspections by foreign countries, nine companies were found to have critical deficiencies.
China says these exchanges can help improve its drug administration, as the country’s strengthening inspection of domestic drugs.

China releases drug inspection report every year from 2016. /CGTN photo
Ding says the latest report shows there were more issues in traditional Chinese medicine and less in vaccines than other drug categories.
"There's a worldwide understanding that if an authority can find more problems, it proves the agency is highly-qualified. Our role is to find problems. Our inspectors are trained to find more problems, and tell the industry to deal with them and prevent them to be found in the future," he says.
The CFDA believes finding problems before drugs are being sold is what's most important in risk control.