Health workers receiving COVID-19 vaccines in Mexico City, Mexico, December 28, 2020. /CFP
Health workers receiving COVID-19 vaccines in Mexico City, Mexico, December 28, 2020. /CFP
With many countries showing keen interest in approving the Chinese COVID-19 vaccines for emergency use, pharmaceutical giants have expedited efforts to start mass production, giving a glimmer of hope to developing countries.
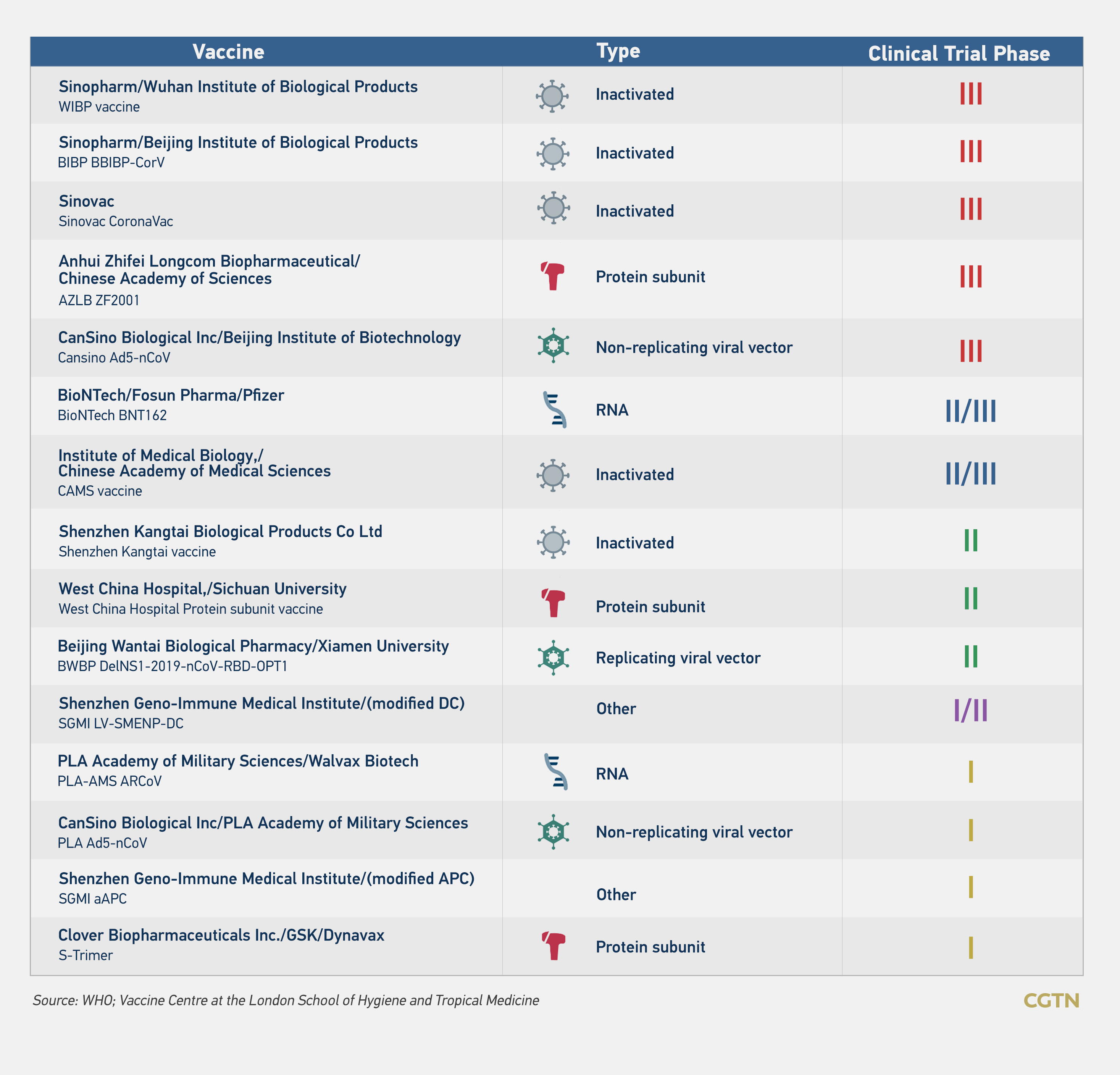
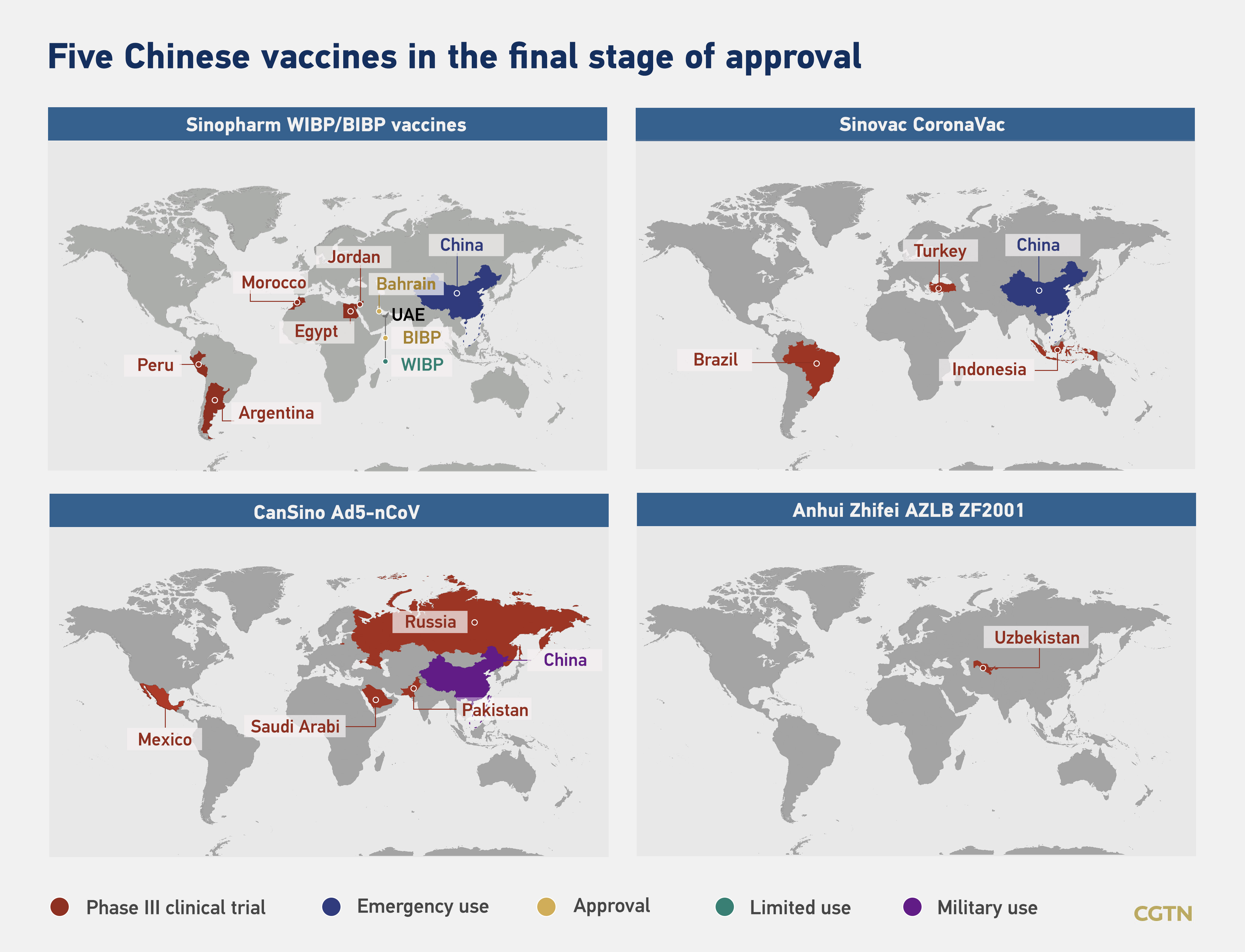
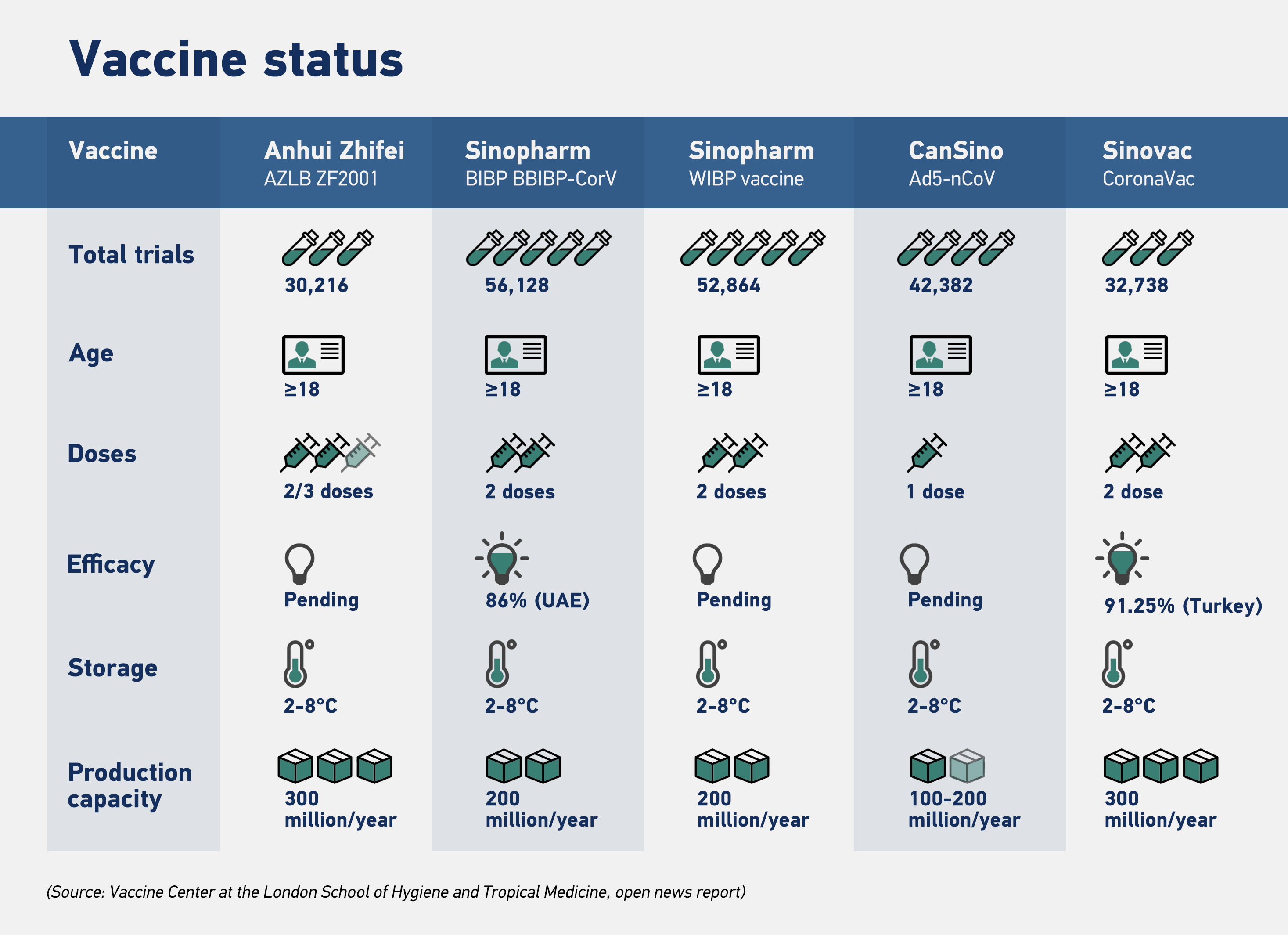
Fifteen COVID-19 vaccines developed by Chinese companies are at various trial stages in more than a dozen countries, including the United Arab Emirates (UAE), Brazil, Pakistan and Peru. Public health experts are closely monitoring five of these candidate vaccines, which are in the final stage of clinical trials.
CGTN Infographic by Du Chenxin
CGTN Infographic by Du Chenxin
They consist of two inactivated vaccines developed by the China National Pharmaceutical Group, which is affiliated with Sinopharm, one inactivated vaccine by Sinovac Biotech Co., one adenoviral vector vaccine jointly prepared by the Beijing Institute of Biotechnology and CanSino, and one recombinant protein vaccine by Anhui Zhifei Longcom Biopharmaceutical Co.
Every vaccine goes through four rigorous stages before it's approved for public use, which includes pre-clinical, phase I, phase II and phase III trials. The results from these trials are submitted to health regulators, which has the final say in approving a vaccine.
"We feel the urgency to develop COVID-19 vaccines not only for Chinese, but also for people across the world," Zhong Nanshan, China's top respiratory expert, said on December 22, adding that the country's vaccines are now in great demand.
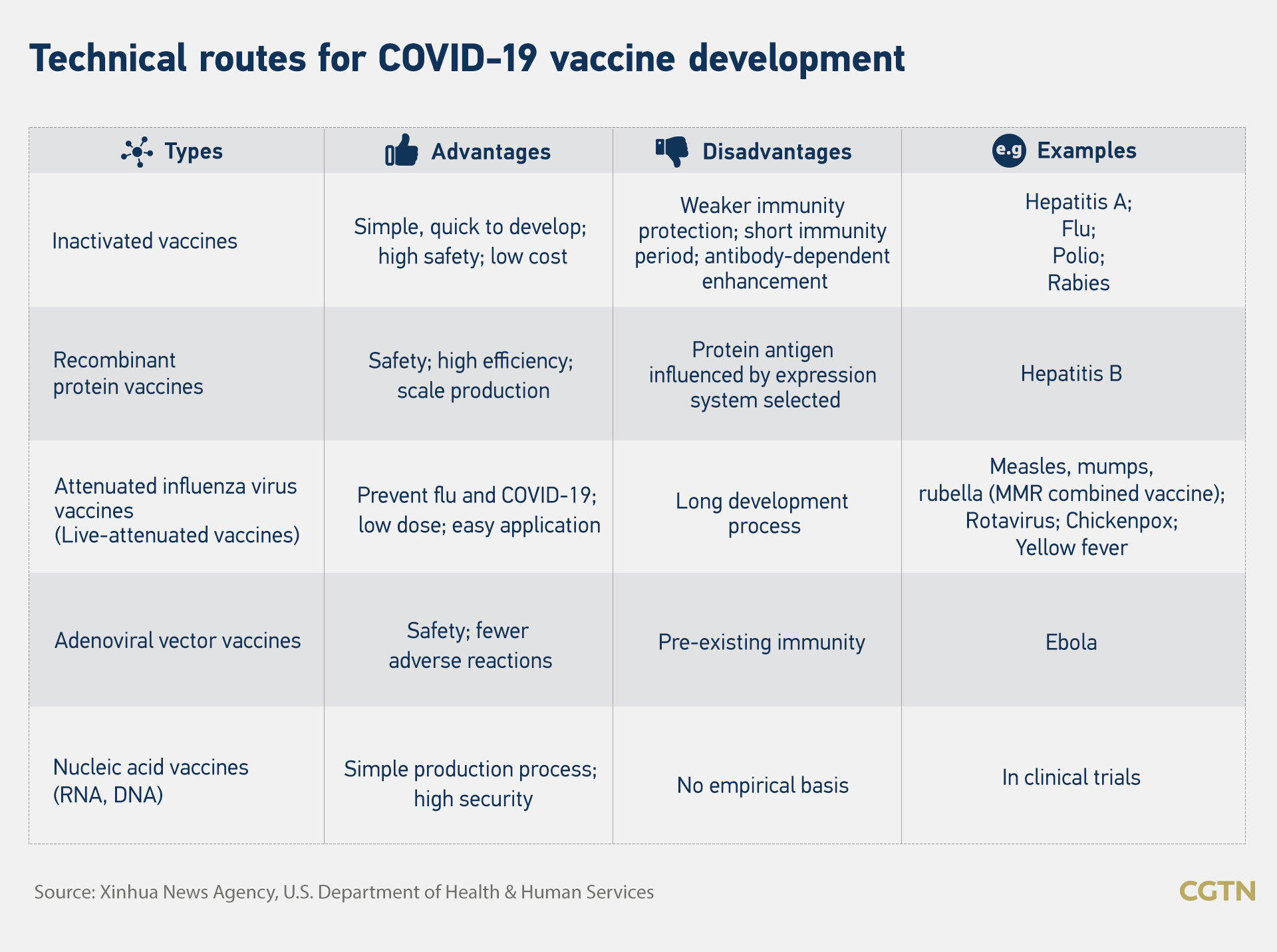
Chinese scientists have started developing vaccines using five different technical approaches to deal with the pandemic since early February. The five technologies, which include a commonly seen inactivated vaccine and an emerging approach DNA and RNA vaccine, have their own advantages and disadvantages.
CGTN Infographic by Du Chenxin
CGTN Infographic by Du Chenxin
"We hope Chinese vaccines will be included in COVAX's procurement list as soon as possible after their development, which will contribute to the accessibility and affordability of vaccines in developing countries," Zhao Lijian, spokesperson of the foreign ministry, told a press briefing in November.
COVAX is a global initiative aimed at working with vaccine manufacturers to provide countries worldwide with equitable access to safe and effective vaccines. But a vast majority of wealthy nations have secured access to Western-developed vaccines. A large share of the vaccine has already been booked by the EU, U.S and UK, leaving a long waiting time for developing countries.
Sinopharm's COVID-19 vaccine, approval by two Arab nations, the UAE and Bahrain, has given much-needed hope for developing countries, while the Russia's approval for another candidate vaccine CanSino has added confidence for more countries.
"The vaccine developed by CanSino is China's first vaccine to be approved by the Russian Ministry of Health to undergo clinical trials in Russia," said Andrey Ivanovich Denisov, the Russian ambassador to China on an annual press conference on Wednesday.
Clinical trials involving about 8,000 volunteers have been completed and the data are being reviewed, the ambassador said, adding the preliminary results are very good.
CGTN Infographic by Li Jingjie, Du Chenxin, Li Yueyun
CGTN Infographic by Li Jingjie, Du Chenxin, Li Yueyun
While Western vaccines, including Pfizer-BioNTech, use the cutting edge mRNA method to build immunity against the novel coronavirus, Sinopharm and Sinovac have adopted the traditional inactivated virus approach in developing the vaccine.
The inactivated virus method has a massive advantage over the mRNA technique as it doesn't require a robust cold chain to preserve the efficacy of the vaccines.
The Pfizer-BioNTech vaccines have to be stored at the arctic temperature of minus 70 degrees Celsius during transit in specially designed shipping containers that "if replenished with dry ice, can be used for up to 15 days; otherwise, specialized freezers costing upwards of $10,000 are needed," according to an article published in Nature. "Once thawed, the companies say, the vaccines can last for up to five days in the fridge."
In contrast, inactivated vaccines can be stored at temperatures ranging from 2-8 degrees Celsius in an ordinary refrigerator.
CGTN Infographic by Li Jingjie
CGTN Infographic by Li Jingjie
For many developing countries, maintaining such ultra-low temperatures for vaccines is a significant challenge because of poor electrification and related infrastructure challenges. Apart from easy handling of inactivated vaccines, Chinese pharmaceutical giants' ability to manufacture vaccines in bulk would help developing countries to inoculate their population rapidly and effectively.
Read more:
China's COVID-19 vaccines: Looking at the concerns and advantages

