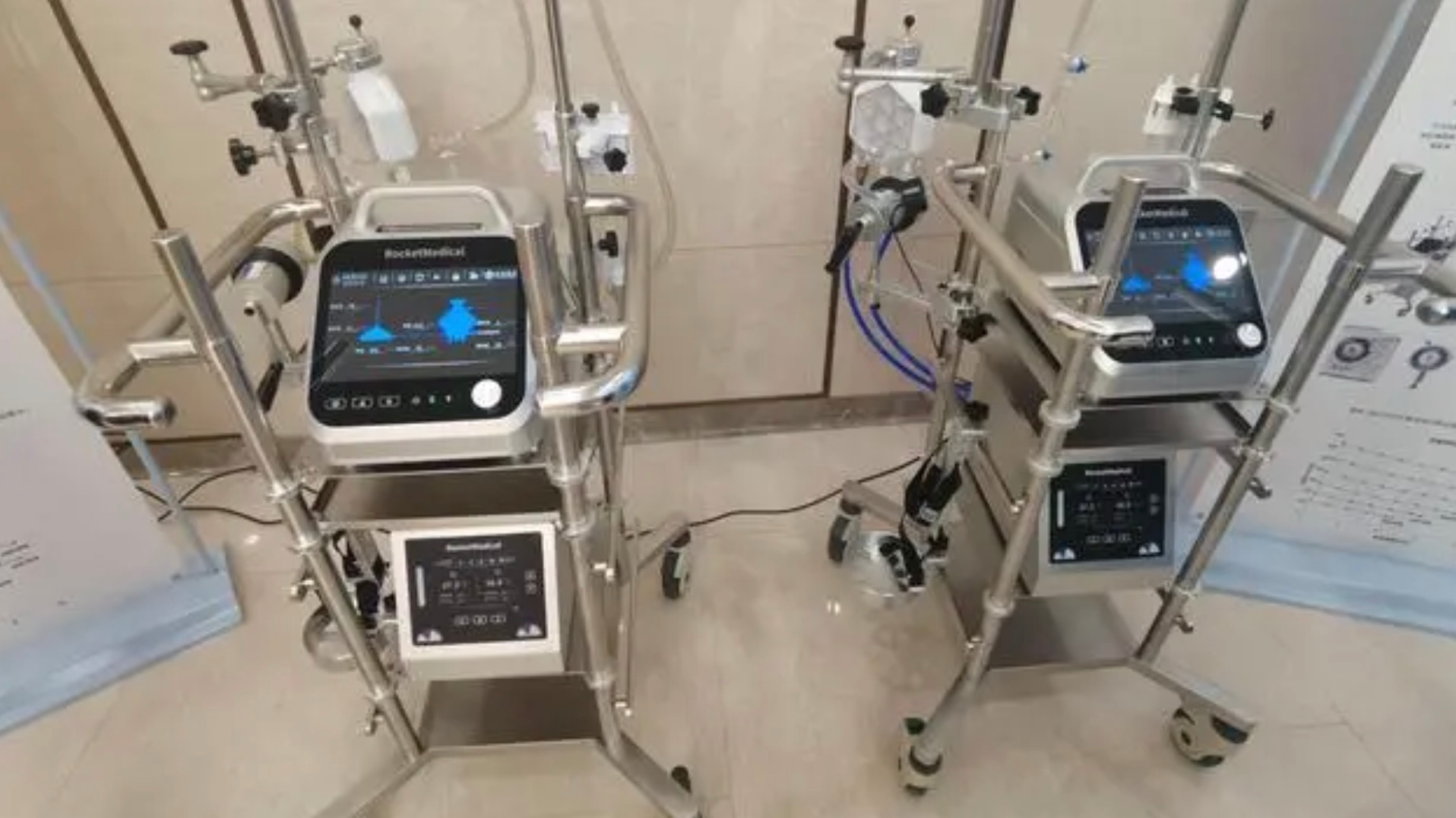
A view of "Revive-I," China's first homegrown extracorporeal membrane oxygenation (ECMO) device, powered by rocket technology. /Xinhua
A view of "Revive-I," China's first homegrown extracorporeal membrane oxygenation (ECMO) device, powered by rocket technology. /Xinhua
China's first domestically-developed extracorporeal membrane oxygenation (ECMO) device "Revive-I," powered by rocket technology, that potentially provides critically ill COVID-19 patients a shot at survival, was launched in Beijing on Wednesday.
An ECMO device is a type of artificial heart and lung machine that provides prolonged cardiac and respiratory support to a patient outside the body.
Typically used in open-heart surgery, the use of ECMOs skyrocketed during the COVID-19 epidemic. It was hailed as a "miracle machine" as reports of severe COVID-19 patients surviving thanks to its use made front-page news.
ECMO with rocket technology
Scientists from Rocket-Medical, a subsidiary company under the China Academy of Launch Vehicle Technology, the country's leading rocket maker, started developing the ECMO machine in 2020 after the COVID-19 outbreak.
The core technology of the ECMO system is derived from a rocket's servomechanism, said Xu Jian, chief engineer of the project.
A servomechanism is a device used to provide control during an operation through the use of feedback. Servo motors on a rocket can help manipulate and fix its positions, directions, and paths during flight.
"ECMO components such as the oxygenator and centrifugal pump involve magnetic levitation and motor control technologies, which work similarly to a rocket's servomechanism," Xu explained.
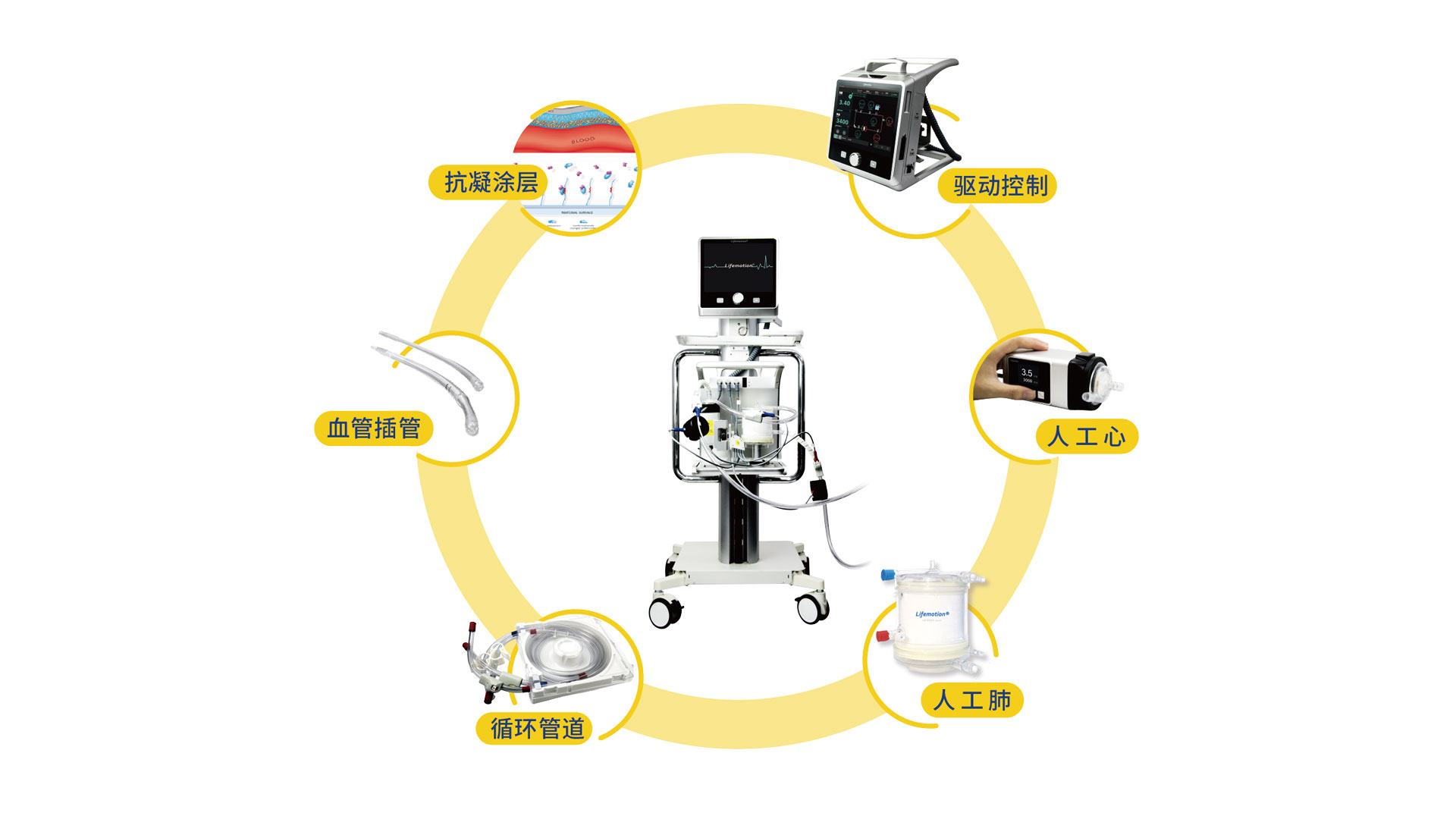
An illustration showing Lifemotion, an ECMO device certified to enter the Chinese market. /Chinabridge Medical Technology
An illustration showing Lifemotion, an ECMO device certified to enter the Chinese market. /Chinabridge Medical Technology
Application and development
Compared with foreign brands, the patented Chinese ECMO is lighter and easily movable, said Zeng Si, director of the research project. It weighs less than 7.5 kg, only one-third of the weight of similar foreign products, and it is light enough to be transported in an ambulance, making it possible to provide ECMO support in emergencies.
Many big hospitals in Beijing and Tianjin took part in the project, offering clinical suggestions and carrying out human trials.
"We used it on seven severe cases. The longest ECMO run time was 25 days," said Weng Li, a clinician at Peking Union Medical College Hospital. "Its operating system is more intuitive."
China's ECMO machine market is dominated by foreign brands. An imported machine is priced between 1.5 million yuan (about $221,300) and 3.5 million yuan. Xu said the price of the Revive-I will be a third to a quarter lower than that of foreign products.
The high cost of an ECMO device usually makes it unaffordable. Generally, the cost of two weeks of ECMO treatment is 100,000 yuan, making it one of the most costly medical procedures.
The domestically-developed ECMO system will offer more access to advanced life support equipment in the country and help reduce the burden of medical treatment for patients, Xu added.
The National Medical Products Administration, China's top drug regulator, has recently issued emergency use authorization for the Revive-I, which is the second domestically-developed ECMO device approved for public sale. The first one was developed by Shenzhen-based Chinabridge Medical.
The COVID-19 epidemic has intensified competition for ECMO manufacturing. Many universities and medical technology companies across China have made investments in ECMO machine development over the past three years.
In September 2020, China's Ministry of Science and Technology announced it would invest 20 million yuan to support domestic research and development.
Read More:
China grants market approval to self-developed ECMO device for severe COVID-19 cases
(With input from Xinhua)