02:05
Editor's note: This is the weekly wrap-up of Chinese COVID-19 vaccines' contribution in the global fight against the virus for April 12-18, 2021. This one also focuses on the recognition of safety and effectiveness that the Chinese vaccines gained around the world.
China has provided anti-epidemic materials to more than 160 countries and international organizations around the world and is providing urgently needed vaccines to more than 100 countries and international organizations, according to Chinese State Councilor and Foreign Minister Wang Yi.
China will continue to make efforts to maintain the stability of the global anti-epidemic materials supply chain and provide support to countries in need, Wang said on April 12.
The safety and effectiveness of Chinese vaccines have earned recognition across the world, with dozens of countries authorizing the use of Chinese vaccines.
Over the last week, Gabonese President Ali Bongo Ondimba and his wife Sylvia Bongo Ondimba received their second doses of the Sinopharm vaccine. On Wednesday, China's Ambassador in Somalia Qin Jian joined officials from the country in receiving their first jabs of the Sinopharm vaccine. Meanwhile, Ukraine started vaccinating its Olympic and Paralympic teams with the Chinese vaccine.
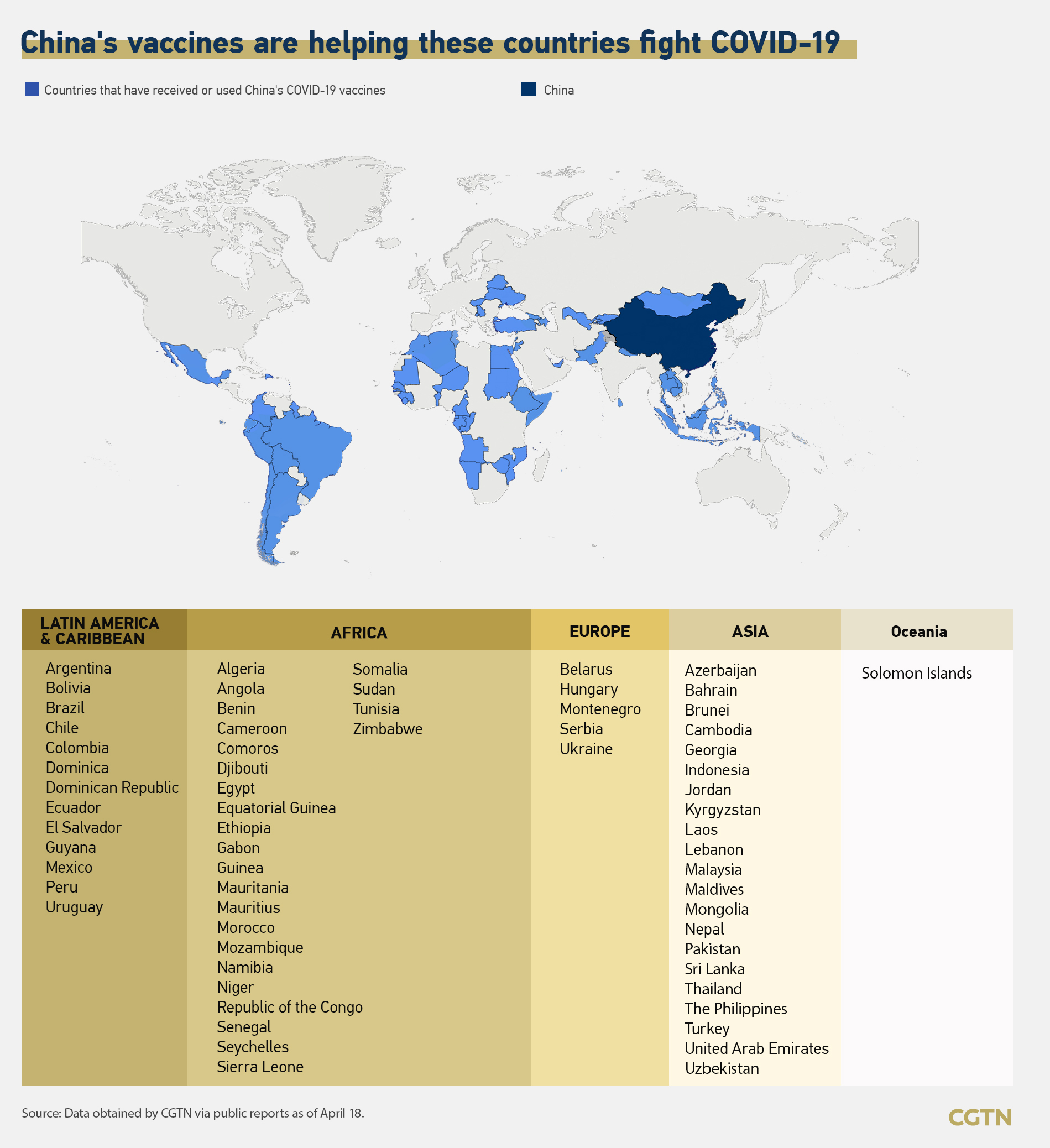
Chile, Turkey, Indonesia and Brazil studies prove Sinovac vaccine's effectiveness
Chile has found that China's Sinovac vaccine provides significant protection against COVID-19 infection and is more effective in preventing severe cases. The Sinovac vaccine is 67 percent effective in preventing symptomatic infection and 80 percent effective in fending off deaths from the disease, according to a report released on Friday by Chile's Ministry of Health.
A report on the efficacy of the vaccine known as CoronaVac also found it 85 percent effective against hospitalizations and 89 percent effective in preventing severe cases.
02:23
The study followed some 10.5 million people in the country who were vaccinated with two doses, one dose, or not vaccinated. It collected the results from those fully vaccinated 14 days after receiving their second dose of the vaccine.
Several other studies have been done on the Sinovac vaccine. Its general efficacy rates range from just above 50 percent in Brazil to 65 percent in Indonesia and over 91 percent in Turkey.
Cameroon, Mauritius and the Solomon Islands receive their first batches of COVID-19 vaccines from China
Cameroon received its first batch of China's Sinopharm COVID-19 vaccines on Sunday, the first COVID-19 vaccine shipment for the country since the start of the pandemic.
00:43

Mauritius also received its first batch of China's Sinopharm vaccines on Tuesday. Mauritian Minister of Foreign Affairs Alan Ganoo hailed the arrival of the Chinese vaccines, saying the country is grateful to the Chinese government for providing vaccines to the country at a difficult time.
The Solomon Islands has received a batch of Chinese-made COVID-19 vaccine on Sunday, the first among Pacific island countries.
Chinese Ambassador to the Solomon Islands Li Ming said at the handover ceremony that the vaccines brought Chinese people's friendship and hope to fight the COVID-19 pandemic and save lives.
"The fact has proved that solidarity and cooperation are humankind's most powerful weapons against the pandemic," Li said, adding that ensuring the equitable distribution of vaccines is essential to developing countries' fight against the pandemic.
"China is the first country to provide COVID-19 vaccines to the Solomon Islands through bilateral channels, which showcased the two countries were trustworthy friends and good partners with sincere cooperation," he added.
China currently has five vaccines in use in its mass immunization campaign, three inactivated-virus vaccines from Sinovac and Sinopharm, a one-shot vaccine from CanSino and one from a team led by Gao Fu, head of the Chinese Center for Disease Control and Prevention, in partnership with Anhui Zhifei Longcom.
The effectiveness of the vaccines ranges from over 50 percent to 79 percent, according to the companies.
Click here to read the previous wrap-up.

